4. The exit gas from an alcohol fermenter is an air-CO mixture containing 10 mol% CO that is to be absorbed in a 5.0

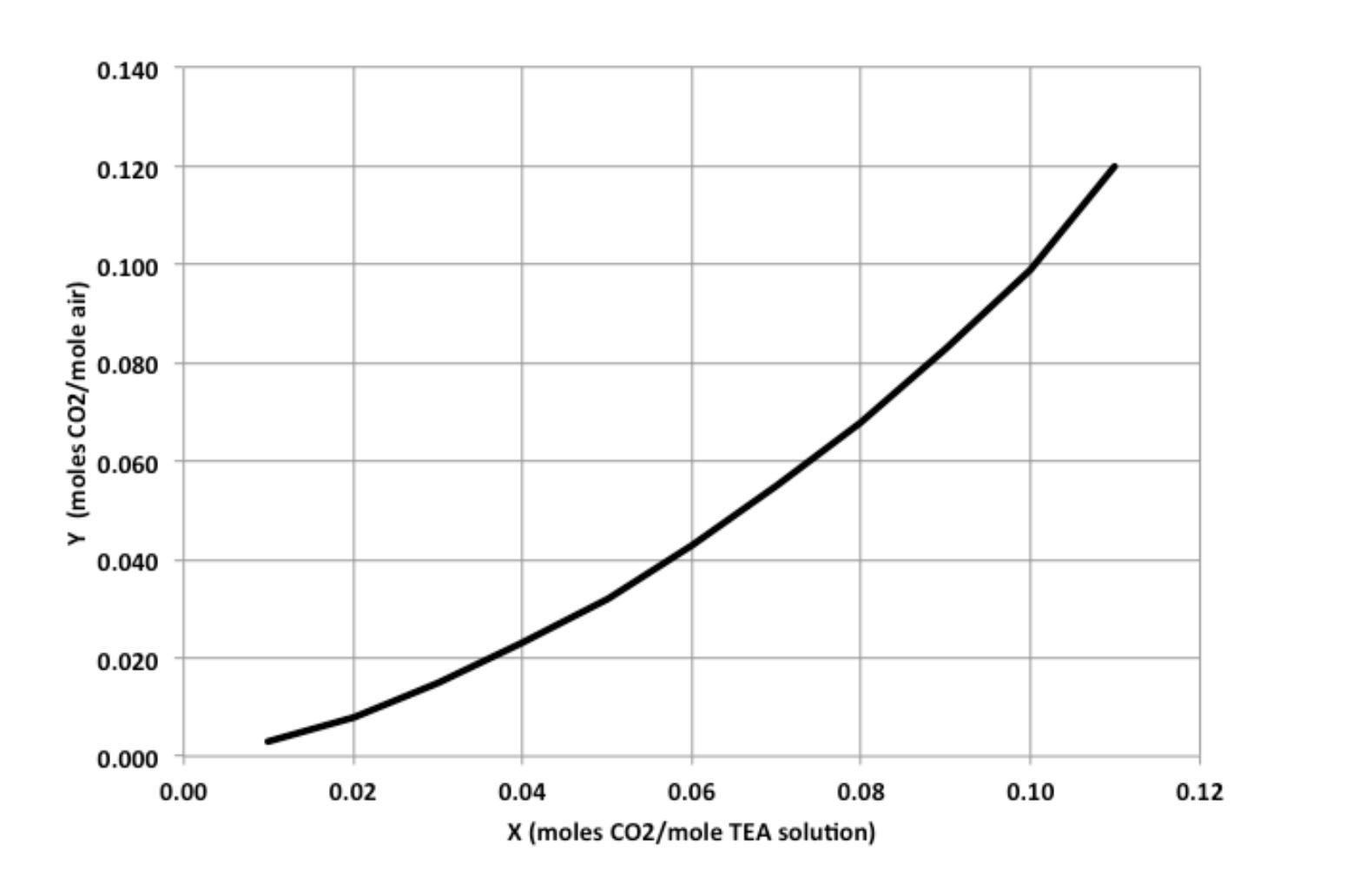
4. The exit gas from an alcohol fermenter is an air-CO mixture containing 10 mol% CO that is to be absorbed in a 5.0 M triethanolamine (TEA) solution containing 0.020 mol CO2/mol TEA. The column operates isothermally at 25 C, and the exit gas is to contain less than 2 mol% CO2. (a). If the tray column is operated at an absorbent flow rate 25% above the minimum required flow rate, but the stage efficiency is only 75% due to diffusion-limited mass transfer in the liquid film, how many trays are required to achieve the desired CO2 separation? (b). If the absorber operates without mass transfer resistance in the liquid film, but at an absorbent flow rate only 10% above the minimum required flow rate, how many trays are required to achieve the desired CO2 separation? (c). Suppose the absorber operates at 35% above the minimum required flow rate with no diffusion limitations, but the TEA solution entering the top of the tray column contains 0.025 mol CO2/mol TEA. How many trays are required in this scenario? Equilibrium data for Y vs. X at 25 C are shown in the plot on the next page, where Y = moles CO2/mole air and X = moles CO2/mole TEA solution. Y (moles CO2/mole air) 0.140 0.120 0.100 0.080 0.060 0.040 0.020 0.000 0.00 0.02 0.04 0.06 0.08 0.10 0.12 X (moles CO2/mole TEA solution)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Lets break down the problem and approach it step by step Problem Summary We need to calculate the number of trays required in a tray column to achieve ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


