Answered step by step
Verified Expert Solution
Question
1 Approved Answer
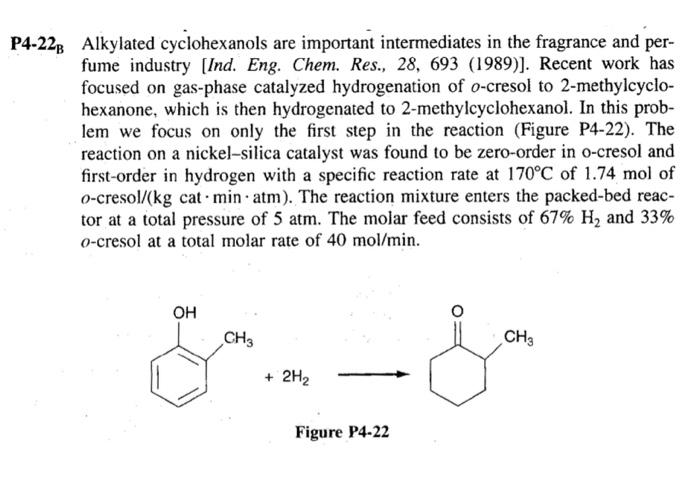
Alkylated cyclohexanols are important intermediates in the fragrance and per- fume industry [Ind. Eng. Chem. Res., 28, 693 (1989)]. Recent work has focused on gas-phase
Alkylated cyclohexanols are important intermediates in the fragrance and per- fume industry [Ind. Eng. Chem. Res., 28, 693 (1989)]. Recent work has focused on gas-phase catalyzed hydrogenation of o-cresol to 2-methylcyclo- hexanone, which is then hydrogenated to 2-methylcyclohexanol. In this prob- lem we focus on only the first step in the reaction (Figure P4-22). The reaction on a nickel-silica catalyst was found to be zero-order in o-cresol and first-order in hydrogen with a specific reaction rate at 170C of 1.74 mol of o-cresol/(kg cat min atm). The reaction mixture enters the packed-bed reac- tor at a total pressure of 5 atm. The molar feed consists of 67% H and 33% o-cresol at a total molar rate of 40 mol/min. OH CH3 + 2H Figure P4-22 CH3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


