Answered step by step
Verified Expert Solution
Question
1 Approved Answer
All please and thank you column directions are next picture 1. Print this worksheet, write on it, then scan to submit. See canvas for instructions
All please and thank you column directions are next picture 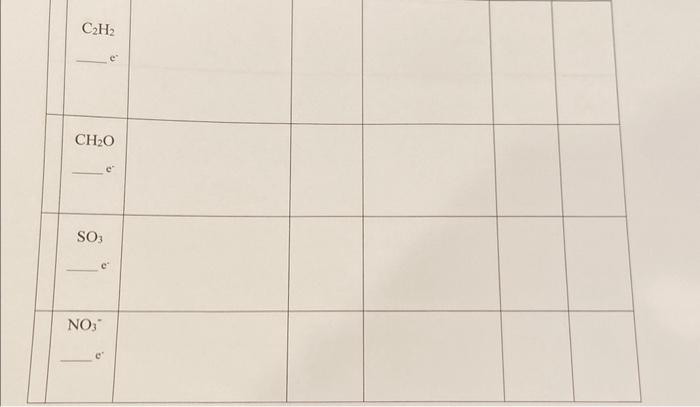

1. Print this worksheet, write on it, then scan to submit. See canvas for instructions on how to scan using your phone for free. If you do not have a smart phone you may take a picture and upload that. 2. Follow the column directions below 3. Determine the molecular geometry of the molecules below, draw the Lewis-Dot structure, if in lab build this structure using modeling kit provided, draw it by hand in 3-D using the wedge/dash convention and determine the polarity. Column 1 Determine the total number of valence electrons in the compound, molecule or ion, and write the number in the space Column 2 Draw the Lewis electron dot structure. Show ALL valence electrons (including lone pairs). Show formal charges on all atoms that have non-zero formal charge. Circle formal charges to distinguish them from the true ion charges. Column 3 Using VSEPR, identify the molecular geometry of the molecule around each central atom. If there is no central atom, write "no central atom" (for example, NaCl ) Column 4 Draw the molecule using wedged and dashed bonds to indicate the 3-D structure. Label all bond angles and any polar bonds. Column 5 Calculate the change in electronegativity for the bonds in the molecule. Indicate bond polarity. Column 6 Using the geometry and the EN, identify whether the compound is polar or nonpolar. Go back to your sketeh in column 4 and add an arrow to indicate the molecular dipole. +5HCl3 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


