Answered step by step
Verified Expert Solution
Question
1 Approved Answer
all questions are related to the info already given. not seperate questions m/forms/d/e/1FAIpQLSdU96J4VmHngejw5JKdddeAdZoft2ZMf14ysbxwOdpk3blzdw/formResponse Knowledge and Understanding Using the following reactions to answer all the following
all questions are related to the info already given. not seperate questions 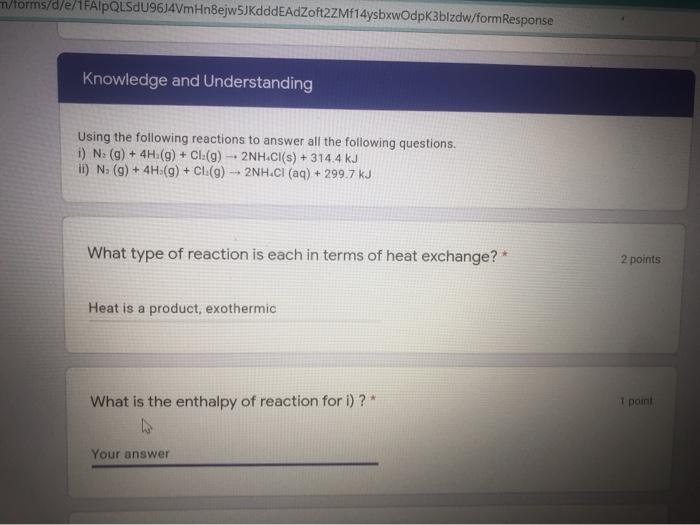
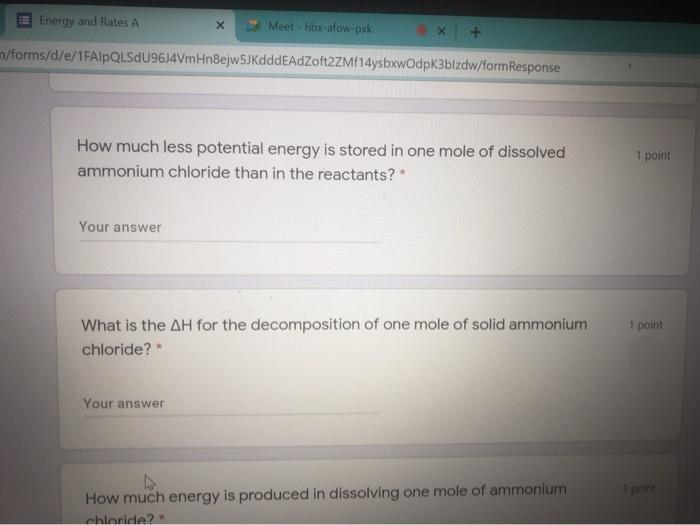
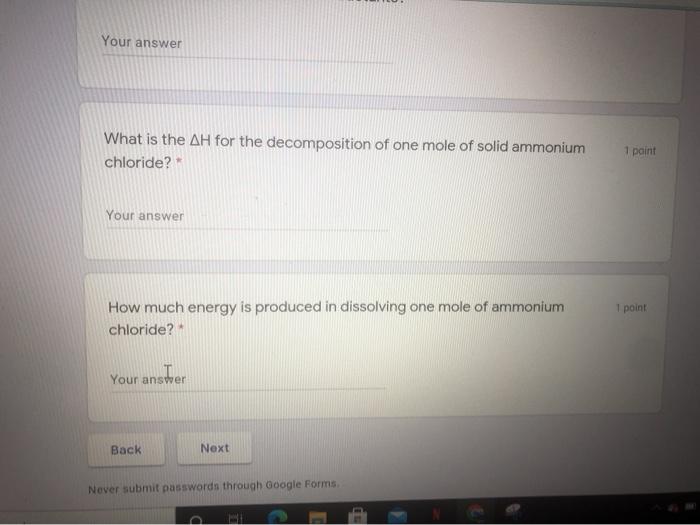
m/forms/d/e/1FAIpQLSdU96J4VmHngejw5JKdddeAdZoft2ZMf14ysbxwOdpk3blzdw/formResponse Knowledge and Understanding Using the following reactions to answer all the following questions. i) N. (9) + 4H (9) + CI:(9) -- 2NH.Cl(s) + 314.4 kJ I) N (9) + 4H:(9) + CL(9) - 2NH.CI (aq) + 299.7 kJ What type of reaction is each in terms of heat exchange?* 2 points Heat is a product, exothermic What is the enthalpy of reaction for i) ?* 1 point Your answer Energy and Rates A Meet - hbx-afow pak n/forms/d/e/1FAIpQLSdU96J4VmHnejw5JKdddeAdZoft2ZMf14ysbxwOdpk3bizdw/formResponse How much less potential energy is stored in one mole of dissolved ammonium chloride than in the reactants? * 1 point Your answer 1 point What is the AH for the decomposition of one mole of solid ammonium chloride? Your answer How much energy is produced in dissolving one mole of ammonium chloride 2 Your answer What is the AH for the decomposition of one mole of solid ammonium chloride? 1 paint Your answer 1 point How much energy is produced in dissolving one mole of ammonium chloride? Your answer ster Back Next Never submit passwords through Google Forms m/forms/d/e/1FAIpQLSdU96J4VmHngejw5JKdddeAdZoft2ZMf14ysbxwOdpk3blzdw/formResponse Knowledge and Understanding Using the following reactions to answer all the following questions. i) N. (9) + 4H (9) + CI:(9) -- 2NH.Cl(s) + 314.4 kJ I) N (9) + 4H:(9) + CL(9) - 2NH.CI (aq) + 299.7 kJ What type of reaction is each in terms of heat exchange?* 2 points Heat is a product, exothermic What is the enthalpy of reaction for i) ?* 1 point Your answer Energy and Rates A Meet - hbx-afow pak n/forms/d/e/1FAIpQLSdU96J4VmHnejw5JKdddeAdZoft2ZMf14ysbxwOdpk3bizdw/formResponse How much less potential energy is stored in one mole of dissolved ammonium chloride than in the reactants? * 1 point Your answer 1 point What is the AH for the decomposition of one mole of solid ammonium chloride? Your answer How much energy is produced in dissolving one mole of ammonium chloride 2 Your answer What is the AH for the decomposition of one mole of solid ammonium chloride? 1 paint Your answer 1 point How much energy is produced in dissolving one mole of ammonium chloride? Your answer ster Back Next Never submit passwords through Google Forms 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


