Answered step by step
Verified Expert Solution
Question
1 Approved Answer
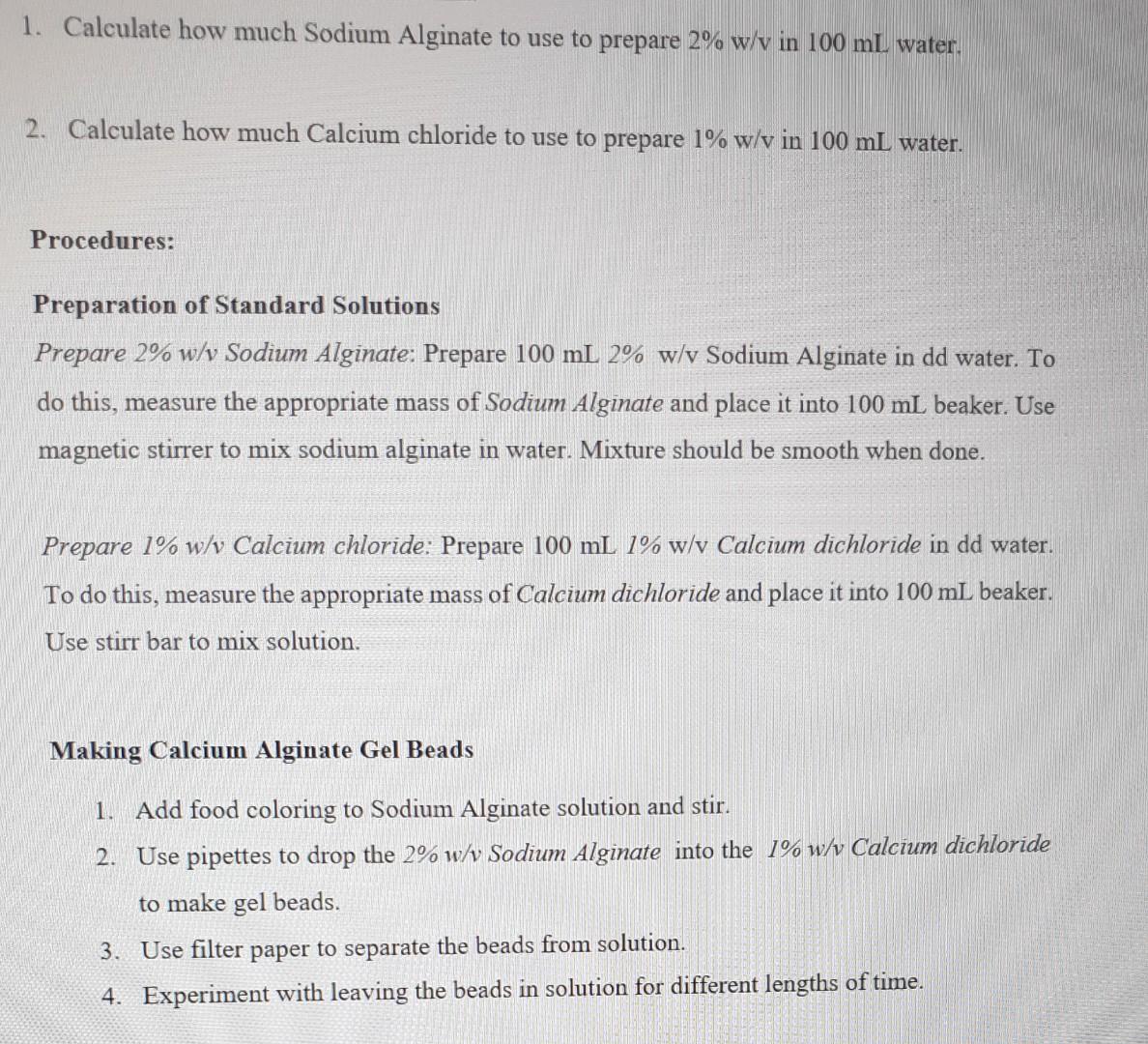
all the questions please 1. Calculate how much Sodium Alginate to use to prepare 2%w/v in 100mL water. 2. Calculate how much Calcium chloride to

all the questions please
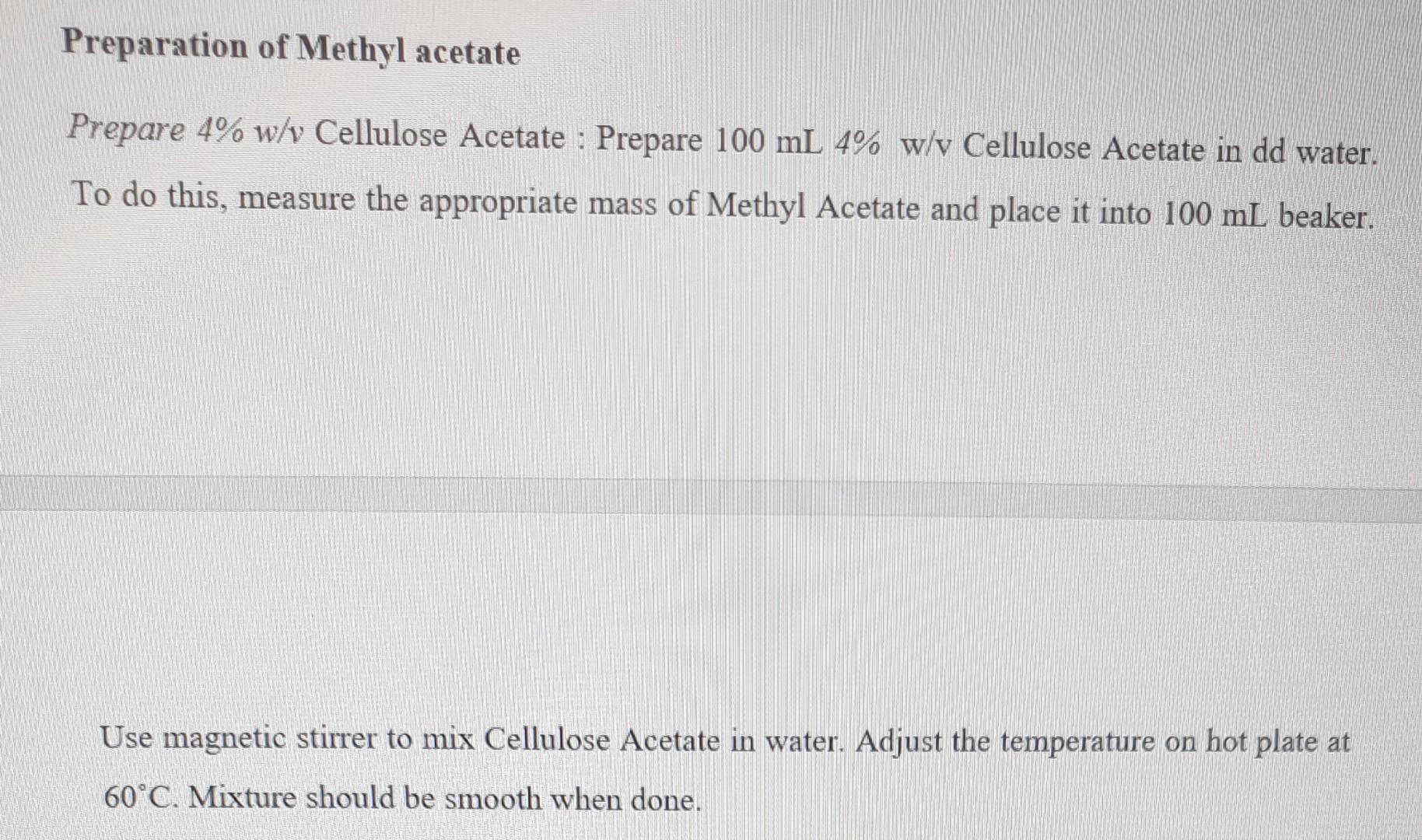
1. Calculate how much Sodium Alginate to use to prepare 2%w/v in 100mL water. 2. Calculate how much Calcium chloride to use to prepare 1%w/v in 100mL water. Procedures: Preparation of Standard Solutions Prepare 2\% w/v Sodium Alginate: Prepare 100mL2%w/v Sodium Alginate in dd water. To do this, measure the appropriate mass of Sodium Alginate and place it into 100mL beaker. Use magnetic stirrer to mix sodium alginate in water. Mixture should be smooth when done. Prepare 1\% w/v Calcium chloride: Prepare 100mL1%w/v Calcium dichloride in dd water. To do this, measure the appropriate mass of Calcium dichloride and place it into 100mL beaker. Use stirr bar to mix solution. Making Calcium Alginate Gel Beads 1. Add food coloring to Sodium Alginate solution and stir. 2. Use pipettes to drop the 2%w/v Sodium Alginate into the 1%w/v Calcium dichloride to make gel beads. 3. Use filter paper to separate the beads from solution. 4. Experiment with leaving the beads in solution for different lengths of time. Preparation of Methyl acetate Prepare 4\% w/v Cellulose Acetate : Prepare 100mL4%w/vCelluloseAcetateinddwater. To do this, measure the appropriate mass of Methyl Acetate and place it into 100mL beaker. Use magnetic stirrer to mix Cellulose Acetate in water. Adjust the temperature on hot plate at 60C. Mixture should be smooth when done. 1. Do all beads form equally? What determines their size and shape? 2. Why is calcium important in the spherification reaction? 3. What is the purpose of adding food coloring to the sodium alginate solution? 4. How would you make 350ml of a 0.20mol/L solution of sulphuric acid from a 18 mol/L stock solution? 5. Two solutions of a substance (non-electrolyte) are mixed in the following manner. 480mL of 1.5M first solution +520mL of 1.2M second solution. What is the molarity of the final mixture? 6. How many grams of sodium dichromate should be added to a 50ml volumetric flask to prepare 0.025 molar sodium dichromate when the flask is filled to mark with waterStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


