Substance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of
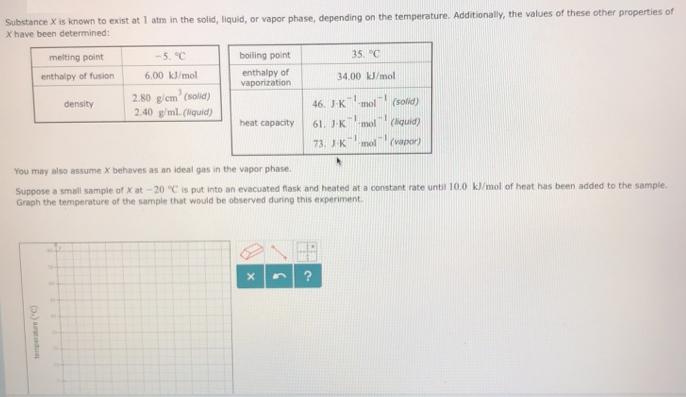
Substance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point -5. C boiling point 35. "C enthalpy of vaporization enthalpy of fusion 6.00 kl/mol 34.00 kJ/mol 2.80 g/em' (solid) 2.40 g/ml. (Nquid) density 46. JK mol (solid) heat capacity 61. J-K mol (liquid) 73. JK mol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Suppose a small sample of X at -20 C is put into an evacuated flask and heated at a constant rate until 10.0 kl/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. Dat
Step by Step Solution
There are 3 Steps involved in it
Step: 1
35C diquid 5C Salid state Salid Liguid vapeur kJlmd med 6...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


