Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Although the actual catalysis in this experiment involves a somewhat complicated reaction mechanism (beyond the scope of our discussion here), you can think of the
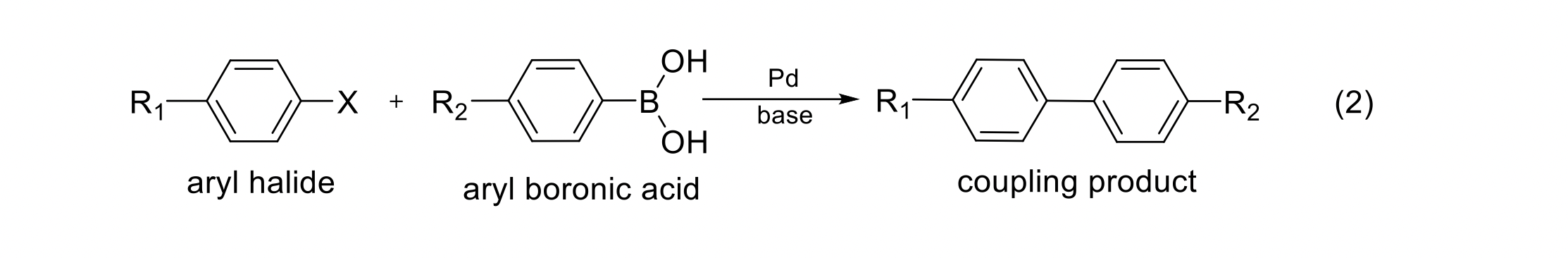
Although the actual catalysis in this experiment involves a somewhat complicated reaction mechanism (beyond the scope of our discussion here), you can think of the net reaction as resembling an SN2-type process with a nucleophile and a leaving group. Using what you know about the reactants and products as shown in Eq. 2 below and the discussion of bond polarities given in the introduction, provide the nucleophile, the specific carbon atom where the substitution takes place, and the leaving group for the net coupling reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


