Answered step by step
Verified Expert Solution
Question
1 Approved Answer
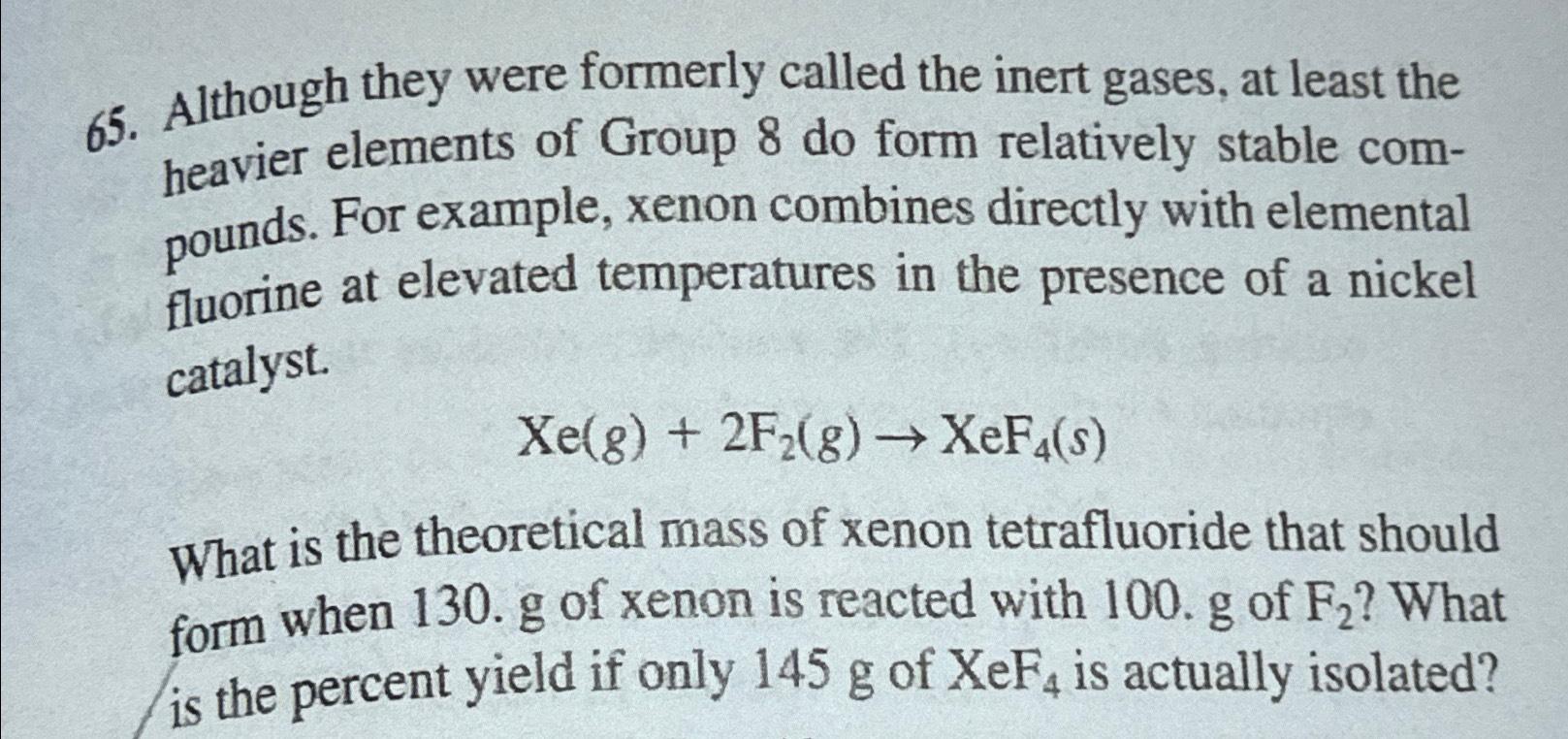
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines
Although they were formerly called the inert gases, at least the heavier elements of Group do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst.
What is the theoretical mass of xenon tetrafluoride that should form when of xenon is reacted with of What is the percent yield if only of is actually isolated?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


