Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Aluminum - lithium alloys are well known in the aerospace industry as strong, light - weight materials for advanced aircraft structures. However, these alloys have
Aluminumlithium alloys are well known in the aerospace industry as strong, lightweight materials for
advanced aircraft structures. However, these alloys have also been considered as electrodes in Liion
batteries. The standard reduction potentials for and are:
NHE
NHE
Thus, in an electrochemical cell with Al metal and Li metal as the two electrodes, lithium will serve as
the anode and aluminum the cathode, with the cathodic reaction written generally as:
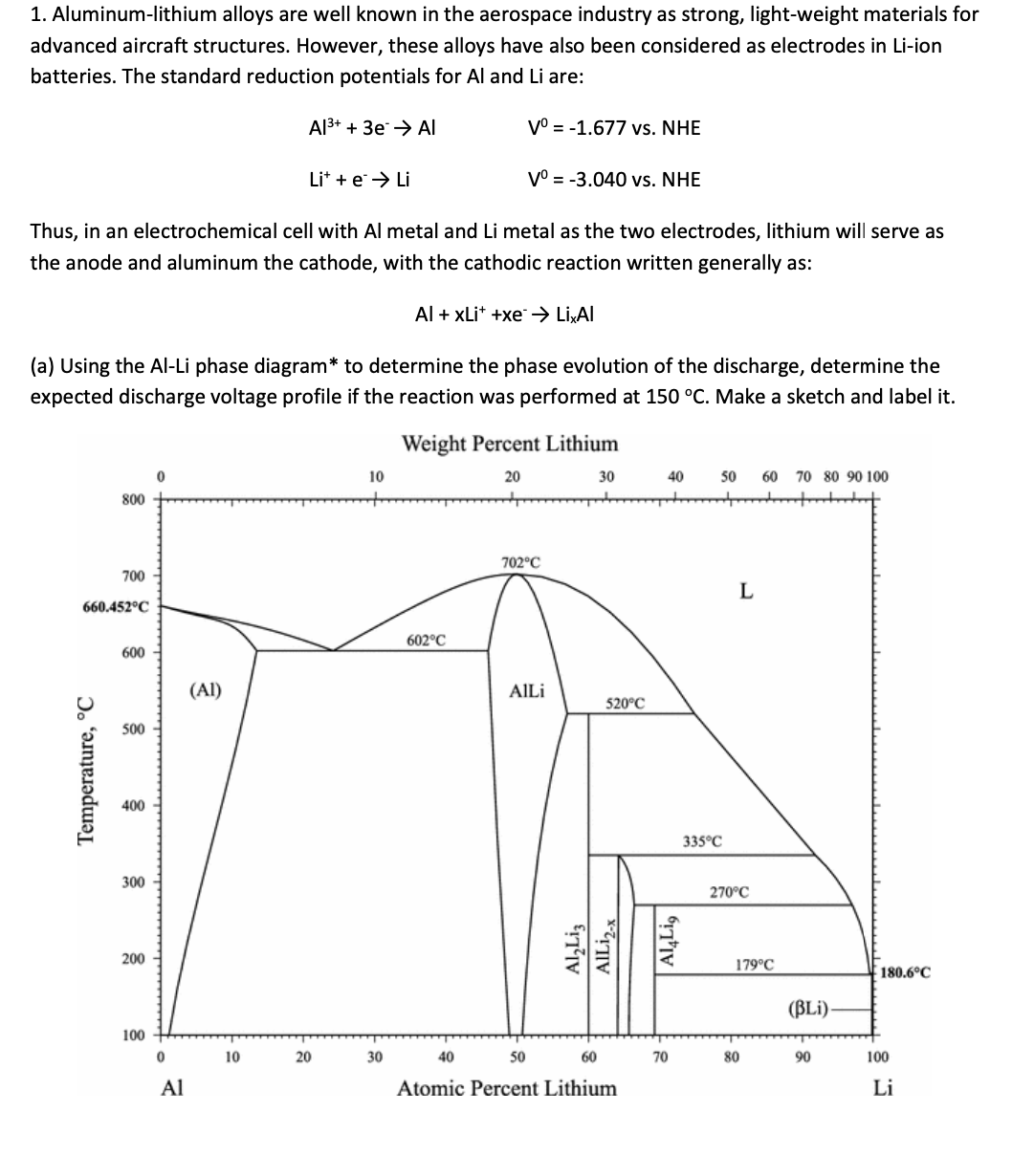
a Using the AlLi phase diagram to determine the phase evolution of the discharge, determine the
expected discharge voltage profile if the reaction was performed at Make a sketch and label it
Weight Percent Lithium
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


