Question
Aluminum metal and bromine liquid (red) react violently to make aluminum bromide (white powder). One way to represent this equilibrium is: 2 Al(s) +
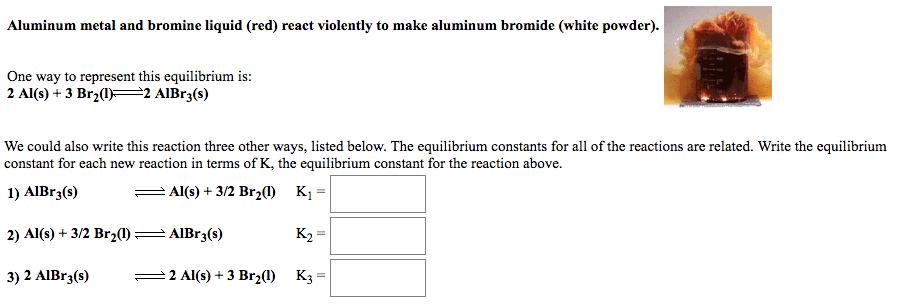
Aluminum metal and bromine liquid (red) react violently to make aluminum bromide (white powder). One way to represent this equilibrium is: 2 Al(s) + 3 Br(1) 2 AlBr3(s) We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) AlBr3(s) Al(s) + 3/2 Br2(1) K = K 2 Al(s) + 3 Br(1) K3= 2) Al(s) + 3/2 Br(1) AlBr3(s) 3) 2 AlBr3(s)
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Packed Bed Reactors Cylindrical vessels filled with a packing material usually inert particles Reactant fluid flows through the void spaces between th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Computer Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



