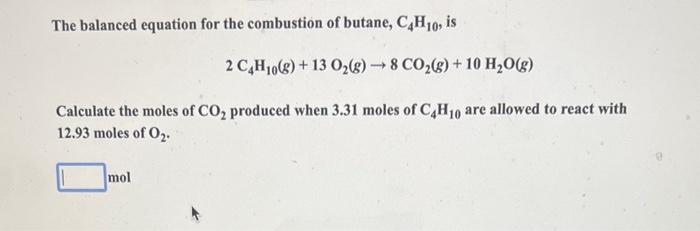
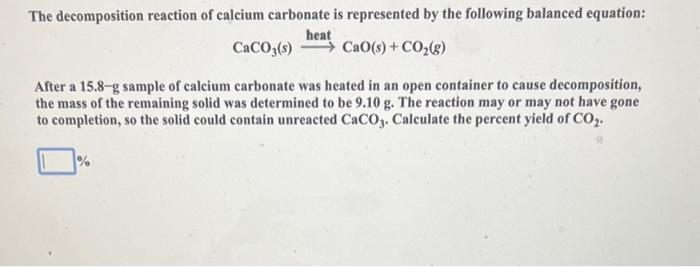
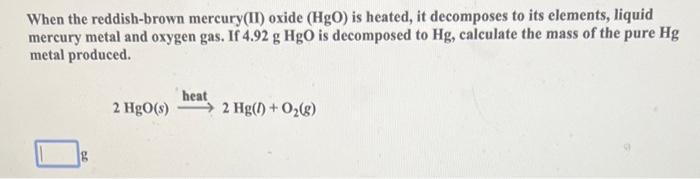Aluminum reacts with chlorine gas according to the following equation shown below. How many moles of Cl2 are required to react with 0.071mol of Al ? 2Al(s)+3Cl2(g)2AlCl3(s) mol When the reddish-brown mercury(II) oxide (HgO) is heated, it decomposes to its elements, liquid mercury metal and oxygen gas. If 4.92gHgO is decomposed to Hg, calculate the mass of the pure Hg metal produced. 2HgO(s)heat2Hg(l)+O2(g) Ethanol, used in alcoholic beverages, can be produced by fermentation of sucrose. The balanced equation for the fermentation process is shown below. What mass of ethanol (C2H5OH) would be produced when 6.31g sucrose reacts by this process? C12H22O11(s)+H2O(l)4C2H5OH(l)+4CO2(g) Enter your answer in the provided box. When mixed, solutions of silver nitrate, AgNO3, and sodium sulfate, Na2SO4, form a precipitate of silver sulfate, Ag2SO4. The balanced equation is: 2AgNO3(aq)+Na2SO4(aq)Ag2SO4(s)+2NaNO3(aq) How many grams of silver sulfate are expected when a solution containing 0.21molAgNO3 is mixed with a solution containing 0.16molNa2SO4 ? The balanced equation for the combustion of butane, C4H10, is 2C4H10(g)+13O2(g)8CO2(g)+10H2O(g) Calculate the moles of CO2 produced when 3.31 moles of C4H10 are allowed to react with 12.93 moles of O2. Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s)+3Cl2(g)2AlCl3(s) Assume that 0.53gAl is mixed with 0.34gCl2. (a) What is the limiting reactant? Cl2 Al (b) What is the maximum amount of AlCl3, in grams, that can be produced? gAlCl3 Sodium bicarbonate reacts with hydrochloric acid in a gas-forming reaction to produce aqueous sodium chloride, water, and carbon dioxide gas: NaHCO3(s)+HCl(aq)NaCl(aq)+H2O(l)+CO2(g) Determine the mass of CO2 gas produced when 8.87g of NaHCO3 is added to a solution that contains 4.03g of HCl. g A student was synthesizing aspirin in the laboratory. Using the amount of limiting reactant, she calculated the mass of aspirin that should form as 10.43g. When she weighed her aspirin product on the balance, its mass was 1.27g. Calculate the percent yield for this synthesis. % CaCO3(s)heatCaO(s)+CO2(g) After a 15.8-g sample of calcium carbonate was heated in an open container to cause decomposition, the mass of the remaining solid was determined to be 9.10g. The reaction may or may not have gone to completion, so the solid could contain unreacted CaCO3. Calculate the percent yield of CO2. Be sure to answer all parts. What is the heat change when 59.4g of water cools from 89.2C to 33.4C ? (The specific heat of water is 4.184J/gC.) 10