Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ammonia is continuously produced in a catalytic reactor through the reaction: N2 + 3H2 2NH3 The reactor is fed with a stream of syngas
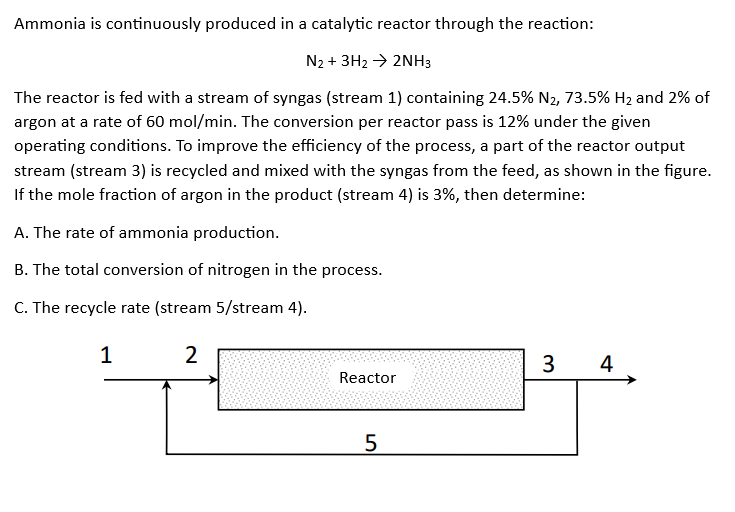
Ammonia is continuously produced in a catalytic reactor through the reaction: N2 + 3H2 2NH3 The reactor is fed with a stream of syngas (stream 1) containing 24.5% N2, 73.5% H2 and 2% of argon at a rate of 60 mol/min. The conversion per reactor pass is 12% under the given operating conditions. To improve the efficiency of the process, a part of the reactor output stream (stream 3) is recycled and mixed with the syngas from the feed, as shown in the figure. If the mole fraction of argon in the product (stream 4) is 3%, then determine: A. The rate of ammonia production. B. The total conversion of nitrogen in the process. C. The recycle rate (stream 5/stream 4). 1 2 3 4 Reactor 5
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


