Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ammonia (NH3) is a metabolite, but is very toxic to aquatic life. NH3 and ammonium (NH4+) exist in equilibrium in an aqueous solution. The
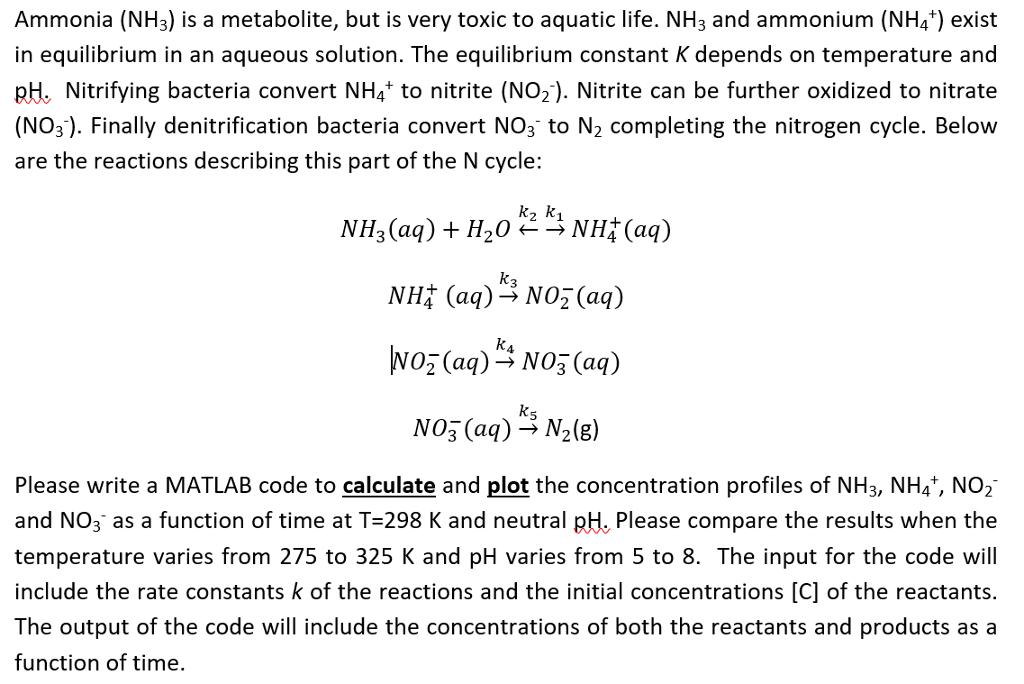
Ammonia (NH3) is a metabolite, but is very toxic to aquatic life. NH3 and ammonium (NH4+) exist in equilibrium in an aqueous solution. The equilibrium constant K depends on temperature and pH. Nitrifying bacteria convert NH4+ to nitrite (NO2). Nitrite can be further oxidized to nitrate (NO3). Finally denitrification bacteria convert NO3 to 2 completing the nitrogen cycle. Below are the reactions describing this part of the N cycle: k NH, (aq) + H,O * * NH (aq) NH (aq)- NOz (aq) K4 NO (aq) + NO3(aq) Ks NO3(aq) N2(g) Please write a MATLAB code to calculate and plot the concentration profiles of NH 3, NH4+, NO and NO3 as a function of time at T=298 K and neutral pH. Please compare the results when the temperature varies from 275 to 325 K and pH varies from 5 to 8. The input for the code will include the rate constants k of the reactions and the initial concentrations [C] of the reactants. The output of the code will include the concentrations of both the reactants and products as a function of time.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


