Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Amounts of proeutectoid ferrite= 268.29 d) The melting temperature of iron= 1147 grams C e) At what carbon concentration do we have the lowest
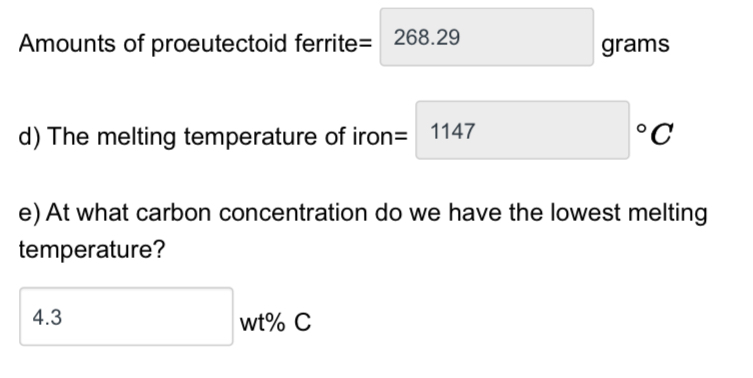
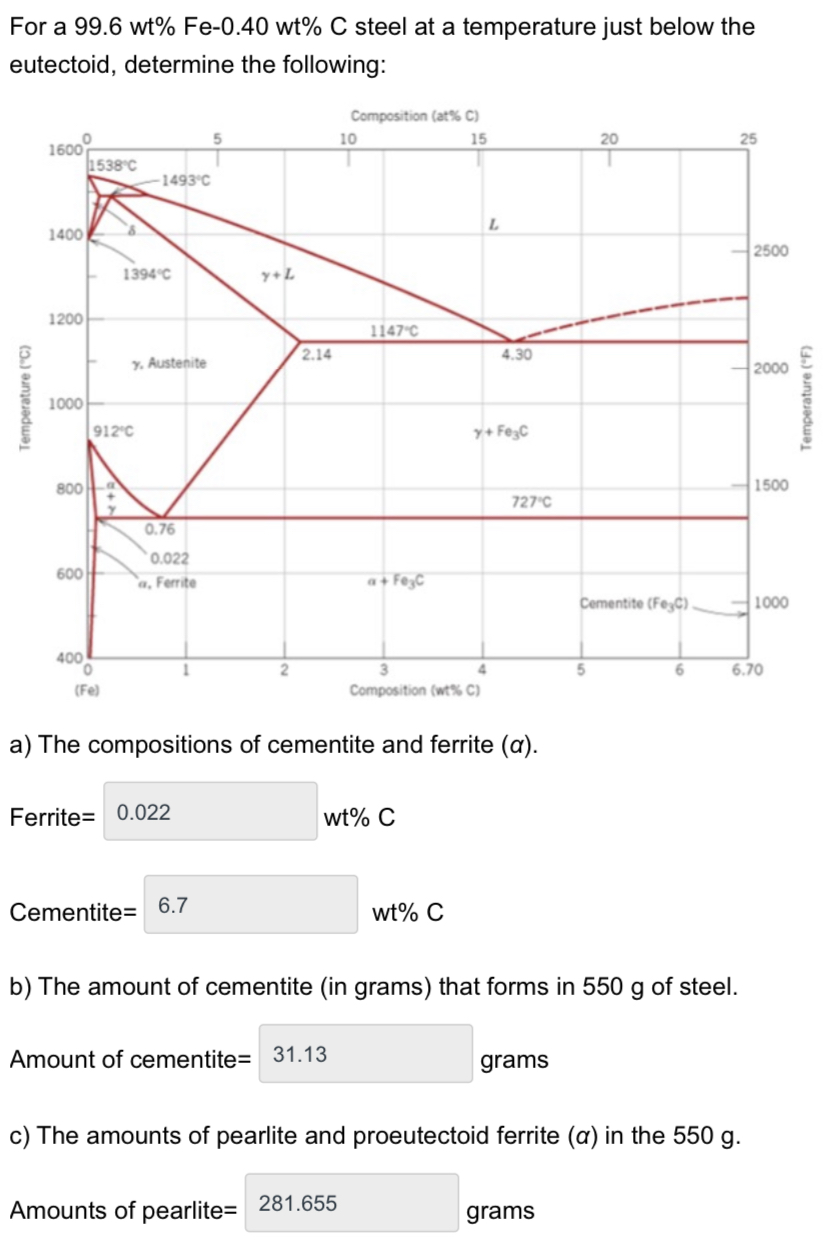
Amounts of proeutectoid ferrite= 268.29 d) The melting temperature of iron= 1147 grams C e) At what carbon concentration do we have the lowest melting temperature? 4.3 wt% C Temperature (C) For a 99.6 wt% Fe-0.40 wt% C steel at a temperature just below the eutectoid, determine the following: Composition (at% C) 5 10 15 20 25 1600 1538C -1493C 1400 1394C Y+L 1200 2500 1147 C y. Austenite 2.14 4.30 2000 1000 912C 800 0.76 0.022 600 a, Ferrite a+ FeyC 400 (Fe) y+ FeC 727C 1500 Cementite (FeyC) 1000 4 5 6 6.70 Composition (wt% C) a) The compositions of cementite and ferrite (a). Ferrite= 0.022 wt% C Cementite= 6.7 wt% C b) The amount of cementite (in grams) that forms in 550 g of steel. Amount of cementite= 31.13 grams c) The amounts of pearlite and proeutectoid ferrite (a) in the 550 g. Amounts of pearlite= 281.655 grams Temperature (F)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


