Answered step by step
Verified Expert Solution
Question
1 Approved Answer
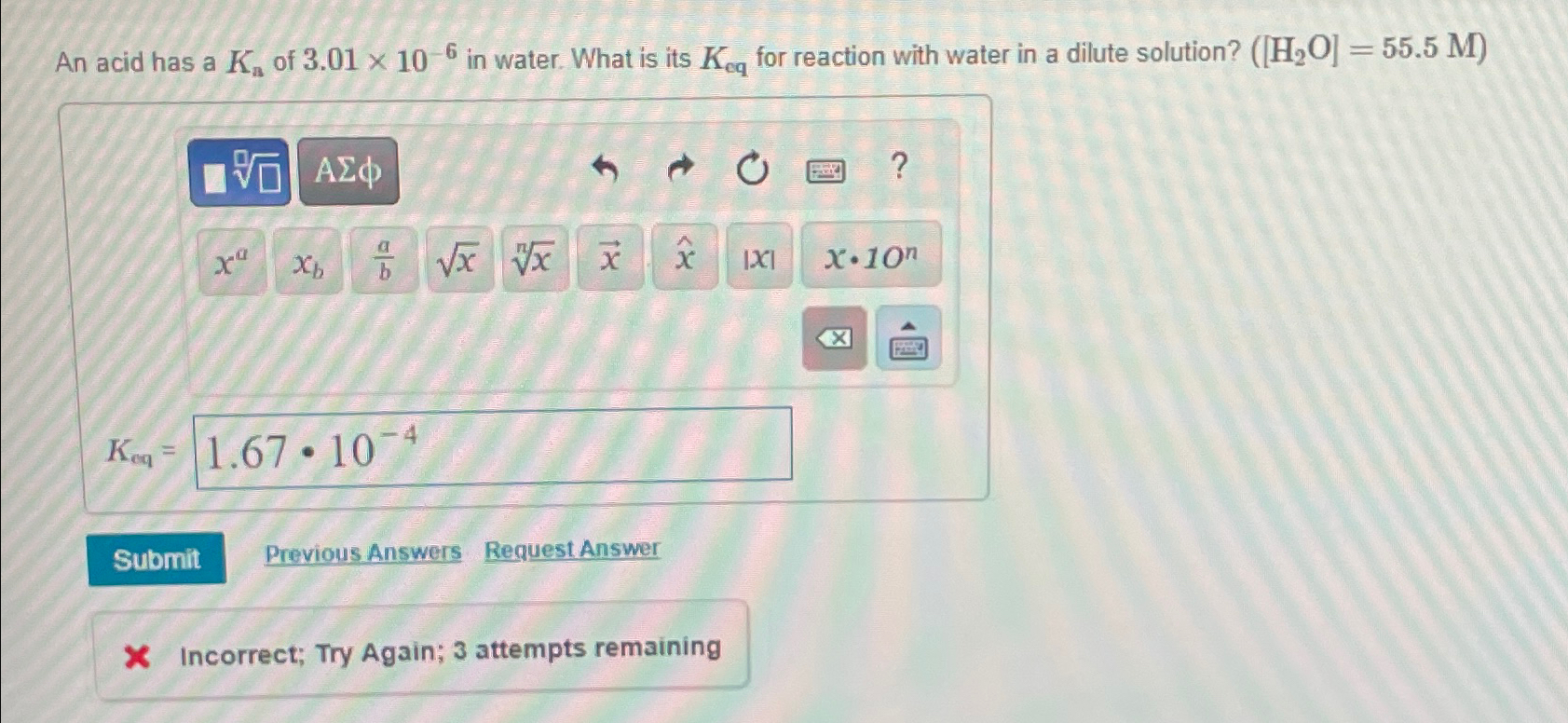
An acid has a K_(a) of 3.01times 10^(-6) in water. What is its K_(eq) for reaction with water in a dilute solution? ([H_(2)O] ) =
An acid has a
K_(a)of
3.01\\\\times 10^(-6)in water. What is its
K_(eq)for reaction with water in a dilute solution?
([H_(2)O])
=(
55.5M)\
K_(eq)=\ Previous Answers Request Answer\ X Incorrect; Try Again; 3 attempts remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


