Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An adiabatic compressor takes air ( ideal gas, C p = 2 9 . 3 J m olK ) at 2 9 0 K and
An adiabatic compressor takes air ideal gas, olK at and bar and delivers air at and bar. What is the entropy change for the air? How much work is required for the steady state compression? If compression from the initial state to bar were done in a totally reversible manner, what would be the final temperature of the compressed gas and how much work would be needed? ANS: olK,
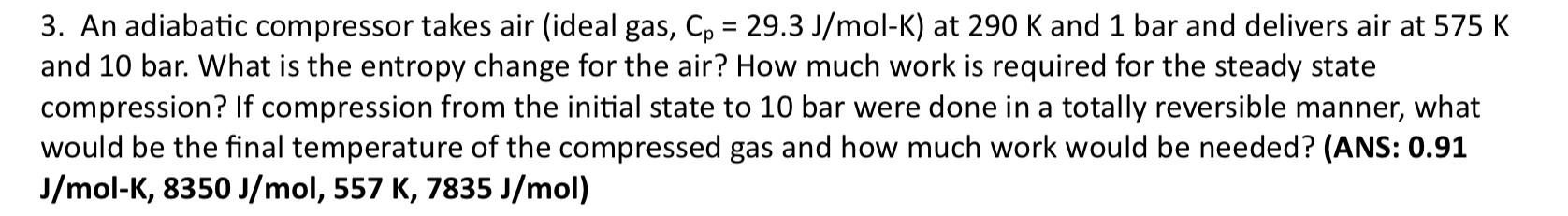
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


