Answered step by step
Verified Expert Solution
Question
1 Approved Answer
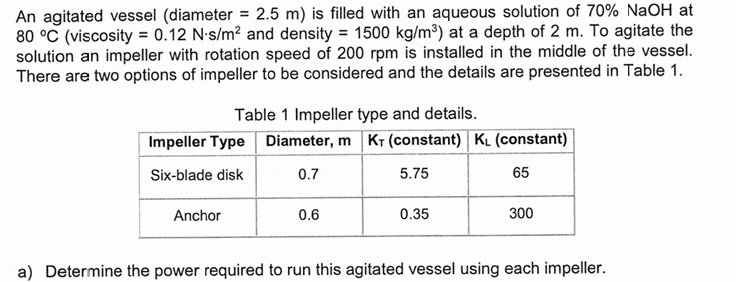
An agitated vessel (diameter =2.5m ) is filled with an aqueous solution of 70%NaOH at 80deg C (viscosity =0.12N*(s)/(m^(2)) and density =1500k(g)/(m^(3)) ) at a
An agitated vessel (diameter
=2.5m) is filled with an aqueous solution of
70%NaOHat\
80\\\\deg C(viscosity
=0.12N*(s)/(m^(2))and density
=1500k(g)/(m^(3))) at a depth of
2m. To agitate the\ solution an impeller with rotation speed of
200rpmis installed in the middle of the vessel.\ There are two options of impeller to be considered and the details are presented in Table 1.\ Table 1 Impeller type and details.\ a) Determine the power required to run this agitated vessel using each impeller.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


