Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An analyst prepares a stock Cr(III)nitrate standard (SSS) by dissolving 1.0000g of dry chromium nitrate into a 1.00 L volumetric flask, and adding 2%
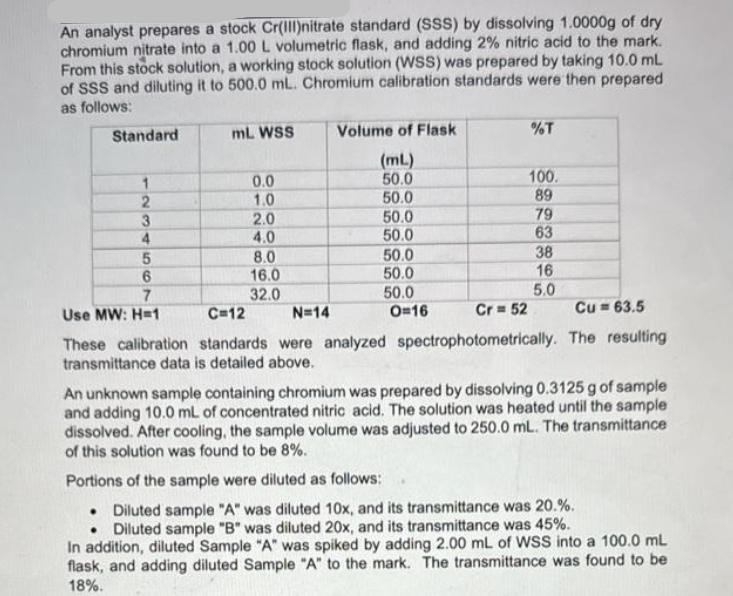

An analyst prepares a stock Cr(III)nitrate standard (SSS) by dissolving 1.0000g of dry chromium nitrate into a 1.00 L volumetric flask, and adding 2% nitric acid to the mark. From this stock solution, a working stock solution (WSS) was prepared by taking 10.0 mL of SSS and diluting it to 500.0 mL. Chromium calibration standards were then prepared as follows: Standard mL. WSS Volume of Flask %T (mL) 1 0.0 50.0 100. 23450 1.0 50.0 89 2.0 50.0 79 4.0 50.0 63 8.0 50.0 38 6 16.0 50.0 16 7 32.0 50.0 5.0 Use MW: H=1 C=12 N=14 O=16 Cr=52 Cu 63.5 These calibration standards were analyzed spectrophotometrically. The resulting transmittance data is detailed above. An unknown sample containing chromium was prepared by dissolving 0.3125 g of sample and adding 10.0 mL of concentrated nitric acid. The solution was heated until the sample dissolved. After cooling, the sample volume was adjusted to 250.0 mL. The transmittance of this solution was found to be 8%. Portions of the sample were diluted as follows: Diluted sample "A" was diluted 10x, and its transmittance was 20.%. Diluted sample "B" was diluted 20x, and its transmittance was 45%. In addition, diluted Sample "A" was spiked by adding 2.00 mL of WSS into a 100.0 mL flask, and adding diluted Sample "A" to the mark. The transmittance was found to be 18%. a. Using this data above, make a properly documented Beer Lambert Plot using Excel. Be sure to provide a trendline, formula and R2 value. What is the linear working range of your calibration curve in concentration? (5 marks) b. Determine the amount of chromium in the unknown solid in ppm and % w/w. (8 marks) c. Did you experience matrix effects? Show all your math.(4 marks) d. Do you have confidence in the results? Explain with detail. (4 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


