Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An equation of state ( EOS ) in thermodynamics, defined narrowly, refers to the two - variable function ( , ) , where is the
An equation of state EOS in thermodynamics, defined narrowly,
refers to the twovariable function where is the
pressure, is the absolute temperature, and is the molar
volume. For example, the equation of state that describes an ideal
gas is is the gas constant:a Convert this integral form of the equation to the
corresponding differential form. In other words, what are the
functions and in the equation below?b If the change in temperature, and the change in molar
volume, are sufficiently small, then the above differential
equation can be used to estimate approximately the change in
pressure:Suppose the absolute temperature increases by and the molar
volume decreases by what is the percentage change of the
pressure?cDetermine the change in when the gas goes from the
state of
to the state of
by integrating your differential equation in a Be specific
about the path you choose to perform the integration. Check that
your answer is the same as if you used the integral form
directly.dThe ideal gas EOS is simple and is a good approximation
at low pressures, but it is often inadequate for real applications.
For a real, nonideal gas, a more accurate EOS is the van der Waals
EOS:where and are constants. Convert this integral
form to the corresponding differential form. Also check that the
differential Pis exact by verifying that:e The constants and are different for different
chemical species. To obtain the constants, we can measure the
critical point of the gas
which is a special state of a gas that satisfies the following
equations:By solving these two equations simultaneously, express and
as functions of and Hint: Relate with the critical molar
volume first, and use the van der Waals EOS to replace with
and
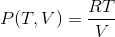
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


