Question
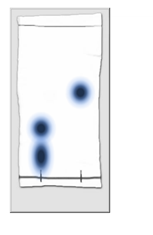
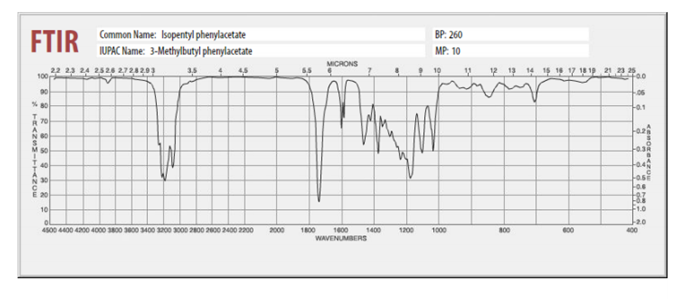
An esterification reaction occurs between 2-phenylacetic acid and 3-methyl-1-butanol in conc. sulfuric acid. Calculate the approximate R f values for both reactants and for the
An esterification reaction occurs between 2-phenylacetic acid and 3-methyl-1-butanol in conc. sulfuric acid.
Calculate the approximate Rf values for both reactants and for the product. Based on the structures of the reactants, which dot/Rf value is associated with which reactant. For the IR, give the approximate position (in cm-1) and the assignment for 2-4 of the most important bands. For example, if you took the IR of the starting alcohol, you might indicate that a strong, broad peak at 3450 cm-1 was observed which can be assigned to the OH stretch.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


