Answered step by step
Verified Expert Solution
Question
1 Approved Answer
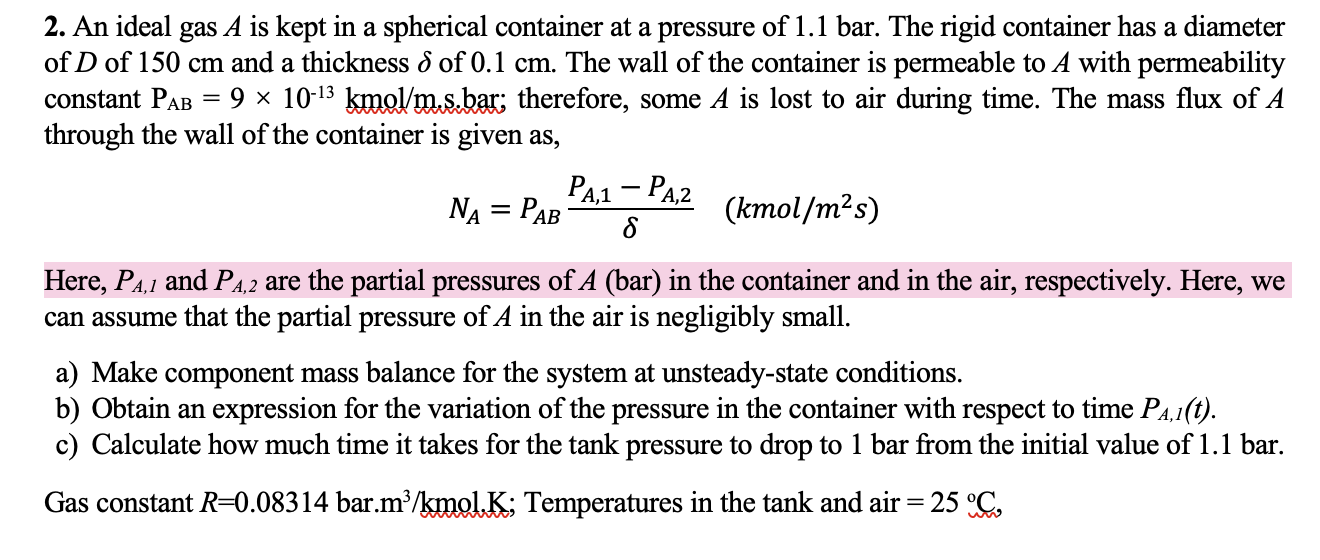
An ideal gas A is kept in a spherical container at a pressure of 1 . 1 bar. The rigid container has a diameter of
An ideal gas is kept in a spherical container at a pressure of bar. The rigid container has a diameter
of of and a thickness of The wall of the container is permeable to A with permeability
constant kmo ; therefore, some is lost to air during time. The mass flux of
through the wall of the container is given as
Here, and are the partial pressures of bar in the container and in the air, respectively. Here, we
can assume that the partial pressure of in the air is negligibly small.
a Make component mass balance for the system at unsteadystate conditions.
b Obtain an expression for the variation of the pressure in the container with respect to time
c Calculate how much time it takes for the tank pressure to drop to bar from the initial value of bar.
Gas constant mol.; Temperatures in the tank and air
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


