Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An ideal plug flow reactor of volume V is operated with recycle at the conditions shown in Figure 2. The reaction A B occurs
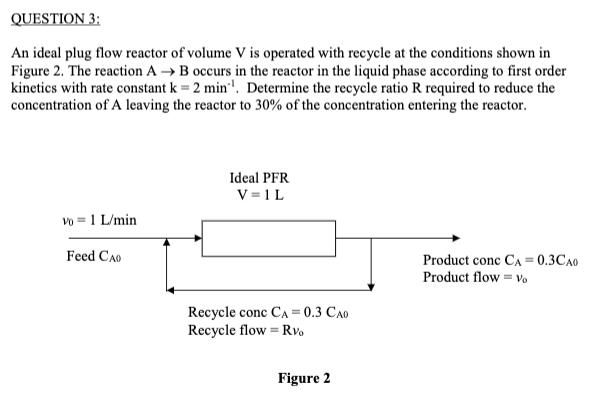 An ideal plug flow reactor of volume V is operated with recycle at the conditions shown in Figure 2. The reaction A → B occurs in the reactor in the liquid phase according to first order kinetics with rate constant k = 2 min-1. Determine the recycle ratio R required to reduce the concentration of A leaving the reactor to 30% of the concentration entering the reactor.
An ideal plug flow reactor of volume V is operated with recycle at the conditions shown in Figure 2. The reaction A → B occurs in the reactor in the liquid phase according to first order kinetics with rate constant k = 2 min-1. Determine the recycle ratio R required to reduce the concentration of A leaving the reactor to 30% of the concentration entering the reactor.
QUESTION 3: An ideal plug flow reactor of volume V is operated with recycle at the conditions shown in Figure 2. The reaction AB occurs in the reactor in the liquid phase according to first order kinetics with rate constant k = 2 min'. Determine the recycle ratio R required to reduce the concentration of A leaving the reactor to 30% of the concentration entering the reactor. Ideal PFR V =1L Vo = 1 L/min Feed CAo Product conc CA = 0.3CA0 %3D Product flow = Vo Recycle conc CA = 0.3 CAo Recycle flow = Rv. Figure 2
Step by Step Solution
★★★★★
3.49 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


