Question
An insulated beaker with negligible mass contains liquid water with a mass of 0.205 kg and a temperature of 83.9 C Part A How
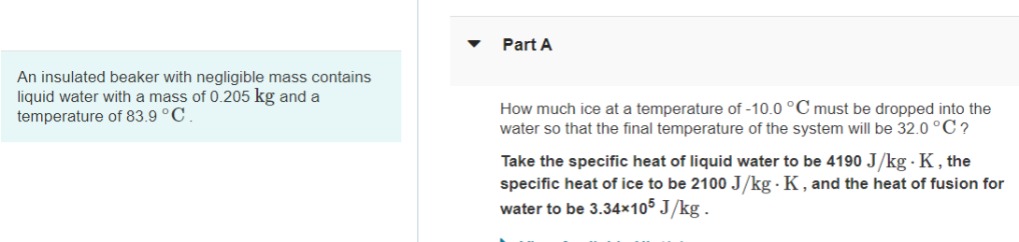
An insulated beaker with negligible mass contains liquid water with a mass of 0.205 kg and a temperature of 83.9 C Part A How much ice at a temperature of -10.0 C must be dropped into the water so that the final temperature of the system will be 32.0 C? Take the specific heat of liquid water to be 4190 J/kg . K, the specific heat of ice to be 2100 J/kg - K, and the heat of fusion for water to be 3.34105 J/kg.
Step by Step Solution
3.48 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
13th edition
321696867, 978-0321696861
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



