Question
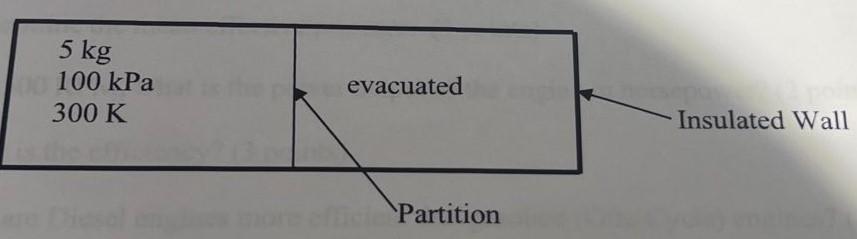
An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 5 kg of an ideal gas at 100
An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 5 kg of an ideal gas at 100 kPa and 300 K. The other part is evacuated. The partition is now pulled out, and the gas expands into the entire tank. (Hint: When applying the 1st Law, be careful where you draw your control volume or control mass)
a) State 1st law and your assumptions. Draw your control volume or control mass. Find the final temperature (K) in the tank.
b) Find the final pressure (kPa) in the tank.
c) Assuming the ideal gas is air, calculate the entropy change (kJ/kg-K). Is this a reversible or irreversible process? Is this process possible?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


