Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1. In an experiment, equal volumes of dilute hydrochloric acid (solution A) and dilute sodium hydrochloric (solution B) of volume 20cm3 are mixed together
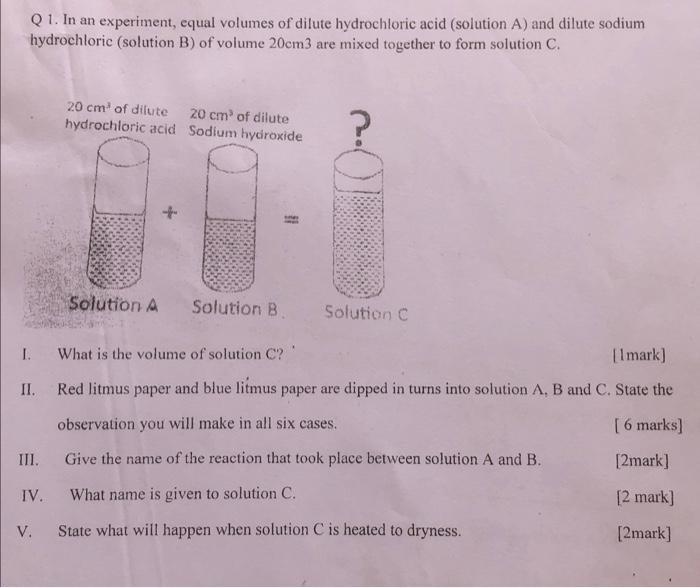
Q1. In an experiment, equal volumes of dilute hydrochloric acid (solution A) and dilute sodium hydrochloric (solution B) of volume 20cm3 are mixed together to form solution C. I. II. III. IV. V. 20 cm of dilute hydrochloric acid Solution A 20 cm of dilute Sodium hydroxide Solution B Solution C What is the volume of solution C? [1 mark] Red litmus paper and blue litmus paper are dipped in turns into solution A, B and C. State the observation you will make in all six cases. [6 marks] Give the name of the reaction that took place between solution A and B. [2mark] What name is given to solution C. [2 mark] State what will happen when solution C is heated to dryness. [2mark]
Step by Step Solution
★★★★★
3.53 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1
3 4 D 20 cm of dilute HCI 20 cm of dilute NaOH a HCI am S 20 mg of HCl meany mol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


