Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Analytically show that the two forms of the equation are equivalent Problem: Clapeyron's ideal gas law, as the name suggests, holds for ideal gases only,
Analytically show that the two forms of the equation are equivalent
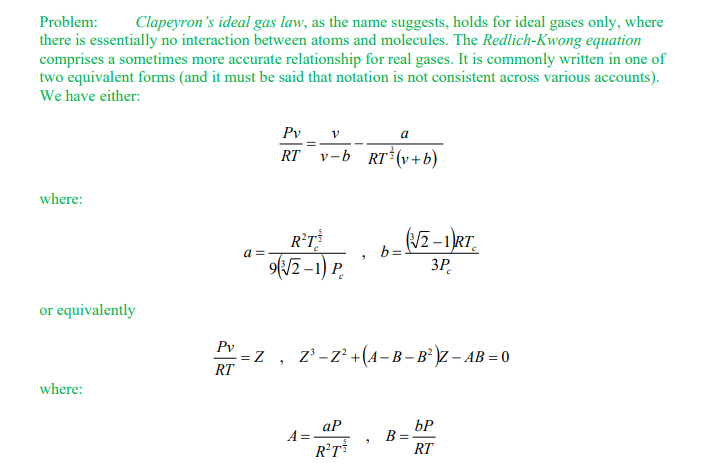
Problem: Clapeyron's ideal gas law, as the name suggests, holds for ideal gases only, where there is essentially no interaction between atoms and molecules. The Redlich-Kwong equation comprises a sometimes more accurate relationship for real gases. It is commonly written in one of two equivalent forms (and it must be said that notation is not consistent across various accounts). We have either: RTPv=vbvRT23(v+b)a where: a=9(321)PcR2Tc25,b=3Pc(321)RTc or equivalently RTPv=Z,Z3Z2+(ABB2)ZAB=0 where: A=R2T25aP,B=RTbPStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


