Answered step by step
Verified Expert Solution
Question
1 Approved Answer
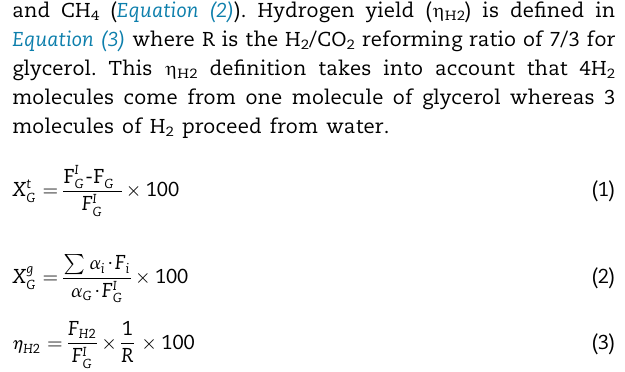
and C H 4 ( Equation ( 2 ) ) . Hydrogen yield ( H 2 ) is defined in Equation ( 3 ) where
and Equation Hydrogen yield is defined in
Equation where is the reforming ratio of for
glycerol. This definition takes into account that
molecules come from one molecule of glycerol whereas
molecules of proceed from water.
Can someone explain to me why the hydrogen yield in this given formula in the study is stoichiometrically adjusted? It was expressed in percent in the study, but I don't understand how to make sense of it I need it to make a material balance equation for a plant design.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


