Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the balanced equation. The reactan at yields the least amount of product is theoretical yield in moles. Converts to grams using the molar mass
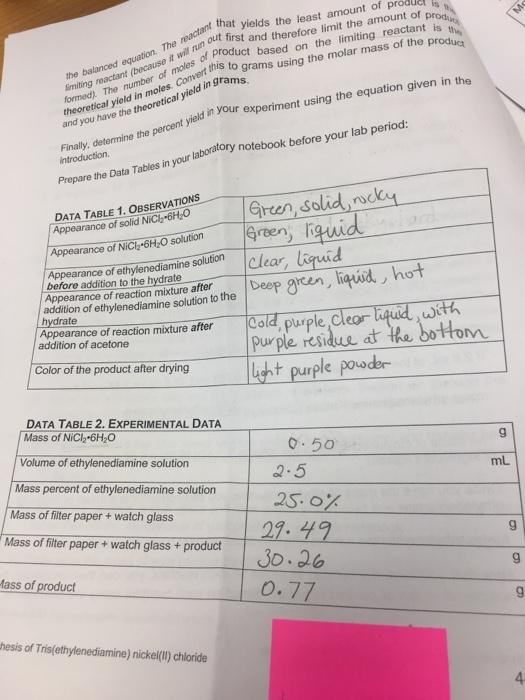

the balanced equation. The reactan at yields the least amount of product is theoretical yield in moles. Converts to grams using the molar mass of the product formed). The number of males of product based on the limiting reactant is the Emiting reactant (because it will run out first and therefore limit the amount of produ and you have the theoretical yield in grams. Finally, determine the percent yield in your experiment using the equation given in the introduction. Prepare the Data Tables in your laboratory notebook before your lab period: DATA TABLE 1. OBSERVATIONS Appearance of solid NiCl-6HO Appearance of NiC6H-O solution Appearance of ethylenediamine solution before addition to the hydrate Appearance of reaction mixture after addition of ethylenediamine solution to the hydrate Appearance of reaction mixture after addition of acetone Color of the product after drying DATA TABLE 2. EXPERIMENTAL DATA Mass of NiCl 6HO Volume of ethylenediamine solution Mass percent of ethylenediamine solution Mass of filter paper + watch glass Mass of filter paper + watch glass + product Mass of product hesis of Tris(ethylenediamine) nickel(II) chloride Green, solid, rocky Green, liquid Clear, liquid Deep green, liquid, hot Cold, purple clear liquid, with Purple residue at the bottom. light purple powder 0.50 2.5 25.0% 29.49 30.26 0.77 9 mL 6 6 st DATA TABLE 3. CALCULATIONS Molar mass of NiCl 6HO Moles of NiCl 6HO used Mass of ethylenediamine solution Mass of ethylenediamine Moles of ethylenediamine Limiting reactant Theoretical Yield of product in moles Molar Mass of the product Theoretical Yield of product in grams Percent yield of product LAB REPORT 237.69108 g/mol 0.0021mol 2.375 g/mol 0.598 D.0098 mol NiC x 6HO A formal lab report is required for this experiment. Include a balanced chemical equation in the Introduction. Show all of your calculations for this experiment including the limiting rea determination. In your Discussion, explain how you can tell which the limiting reactant i explain how your calculations help you to determine the limiting reactar For your Error Analysis, think through carefully, step by step, what you unavoidable experimental errors could have been made.
Step by Step Solution
★★★★★
3.38 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Introduction The balanced chemical equation for the synthesis of Trisethylenediamine nickelII chlori...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


