Question
Anlsysis of a given fuel has a equivalent molar composition of C6.2H14.305.1 Determine the mass of air required for stoichiometric combustion with 1 kg
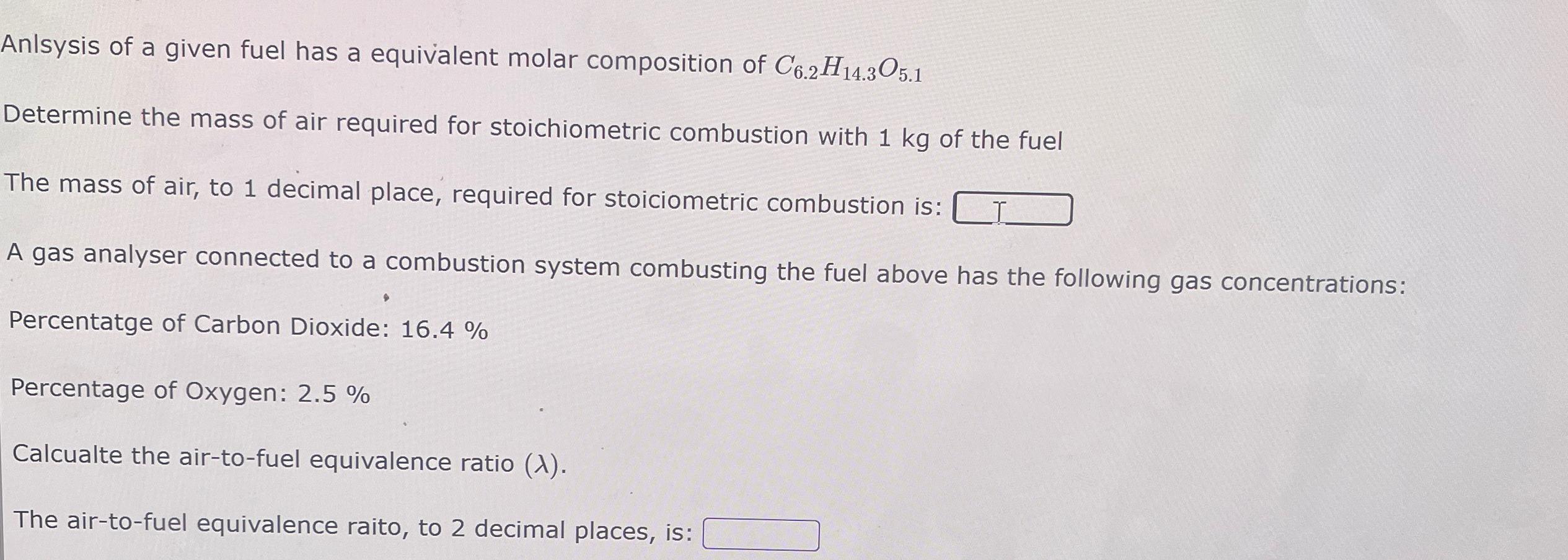
Anlsysis of a given fuel has a equivalent molar composition of C6.2H14.305.1 Determine the mass of air required for stoichiometric combustion with 1 kg of the fuel The mass of air, to 1 decimal place, required for stoiciometric combustion is: A gas analyser connected to a combustion system combusting the fuel above has the following gas concentrations: Percentatge of Carbon Dioxide: 16.4 % Percentage of Oxygen: 2.5 % Calcualte the air-to-fuel equivalence ratio (X). The air-to-fuel equivalence raito, to 2 decimal places, is: 10
Step by Step Solution
3.24 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Solutions For stoichiometrically correct combustion Number ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
11th Edition
1305580346, 978-1305580343
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



