Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ans is 1 / 3 A , . 2 6 6 7 B , . 4 C mol frac in the feed, prove why please
ans is ABC mol frac in the feed, prove why please
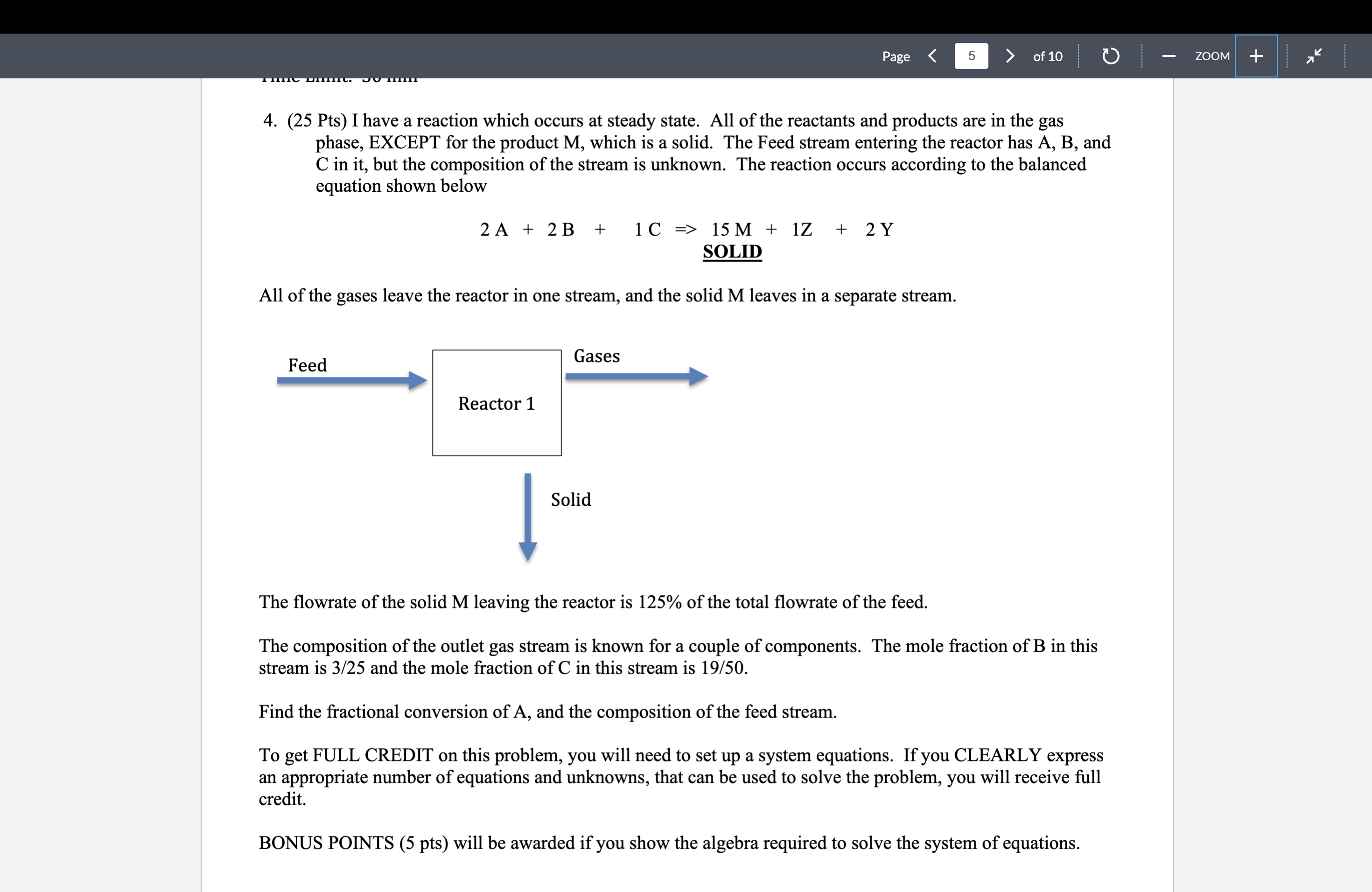
Pts I have a reaction which occurs at steady state. All of the reactants and products are in the gas
phase, EXCEPT for the product which is a solid. The Feed stream entering the reactor has A B and
in it but the composition of the stream is unknown. The reaction occurs according to the balanced
equation shown below
SOLID
All of the gases leave the reactor in one stream, and the solid leaves in a separate stream.
The flowrate of the solid leaving the reactor is of the total flowrate of the feed.
The composition of the outlet gas stream is known for a couple of components. The mole fraction of B in this
stream is and the mole fraction of in this stream is
Find the fractional conversion of and the composition of the feed stream.
To get FULL CREDIT on this problem, you will need to set up a system equations. If you CLEARLY express
an appropriate number of equations and unknowns, that can be used to solve the problem, you will receive full
credit.
BONUS POINTS pts will be awarded if you show the algebra required to solve the system of equations.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


