Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer a b and c as briefly as possible An unknown gas, D, reacts with fluorine gas to form the compound DF4(9), as represented by
answer a b and c as briefly as possible 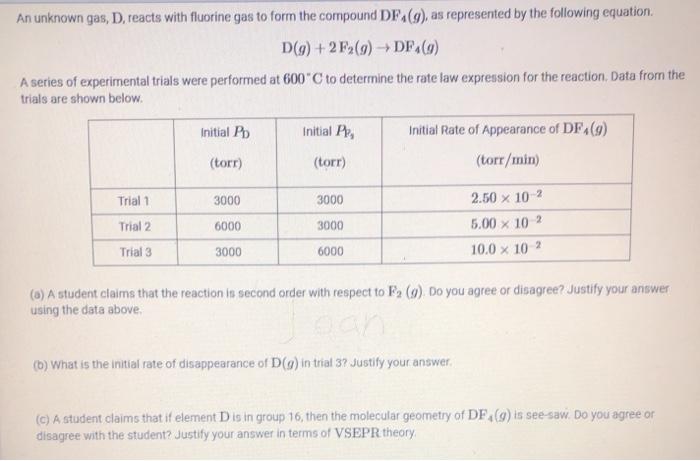
An unknown gas, D, reacts with fluorine gas to form the compound DF4(9), as represented by the following equation D(g) + 2F(9) DF(9) A series of experimental trials were performed at 600C to determine the rate law expression for the reaction, Data from the trials are shown below Initial Pb Initial P, Initial Rate of Appearance of DF (9) (torr) (torr) (torr/min) Trial 1 3000 3000 Trial 2 6000 3000 2.50 x 102 5.00 x 102 10.0 x 102 Trial 3 3000 6000 (a) A student claims that the reaction is second order with respect to F, (w). Do you agree or disagree? Justify your answer using the data above (6) What is the initial rate of disappearance of D() in trial 3? Justify your answer (C) A student claims that if element D is in group 16, then the molecular geometry of DF (9) is see-saw. Do you agree or disagree with the student? Justify your answer in terms of VSEPR theory 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


