Answered step by step
Verified Expert Solution
Question
1 Approved Answer
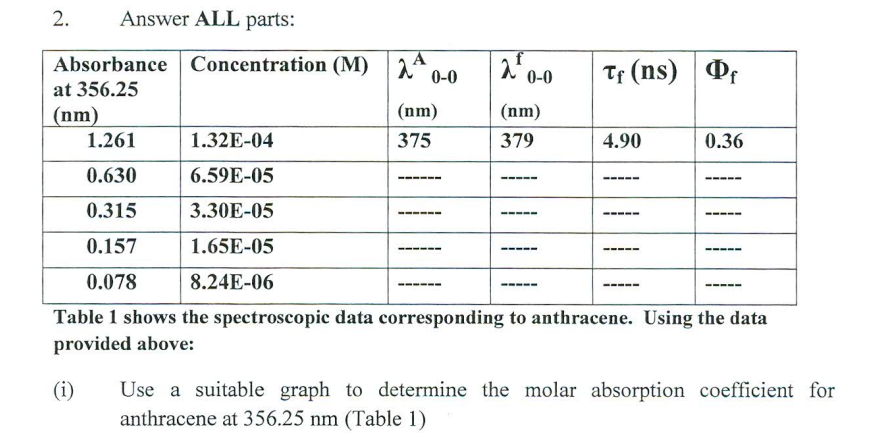
answer all parts 2. Answer ALL parts: Table 1 shows the spectroscopic data corresponding to anthracene. Using the data provided above: (i) Use a suitable
answer all parts
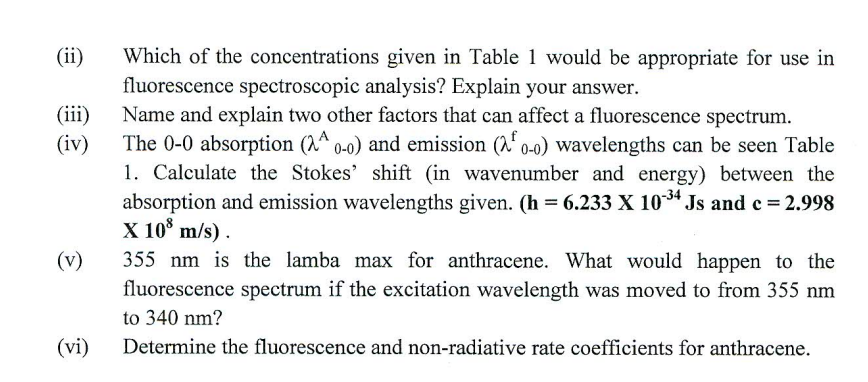
2. Answer ALL parts: Table 1 shows the spectroscopic data corresponding to anthracene. Using the data provided above: (i) Use a suitable graph to determine the molar absorption coefficient for anthracene at 356.25nm (Table 1) (ii) Which of the concentrations given in Table 1 would be appropriate for use in fluorescence spectroscopic analysis? Explain your answer. (iii) Name and explain two other factors that can affect a fluorescence spectrum. (iv) The 00 absorption (A00) and emission (00f) wavelengths can be seen Table 1. Calculate the Stokes' shift (in wavenumber and energy) between the absorption and emission wavelengths given. (h=6.2331034Js and c=2.998 X 108m/s ) . (v) 355nm is the lamba max for anthracene. What would happen to the fluorescence spectrum if the excitation wavelength was moved to from 355nm to 340nm ? (vi) Determine the fluorescence and non-radiative rate coefficients for anthracene. 2. Answer ALL parts: Table 1 shows the spectroscopic data corresponding to anthracene. Using the data provided above: (i) Use a suitable graph to determine the molar absorption coefficient for anthracene at 356.25nm (Table 1) (ii) Which of the concentrations given in Table 1 would be appropriate for use in fluorescence spectroscopic analysis? Explain your answer. (iii) Name and explain two other factors that can affect a fluorescence spectrum. (iv) The 00 absorption (A00) and emission (00f) wavelengths can be seen Table 1. Calculate the Stokes' shift (in wavenumber and energy) between the absorption and emission wavelengths given. (h=6.2331034Js and c=2.998 X 108m/s ) . (v) 355nm is the lamba max for anthracene. What would happen to the fluorescence spectrum if the excitation wavelength was moved to from 355nm to 340nm ? (vi) Determine the fluorescence and non-radiative rate coefficients for anthracene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


