Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ANSWER ALL PARTS OF THE SINGLE QUESTION (A)-(D) Solve the following modified version of R&E 6.18. We wish to convert the CO in a waste
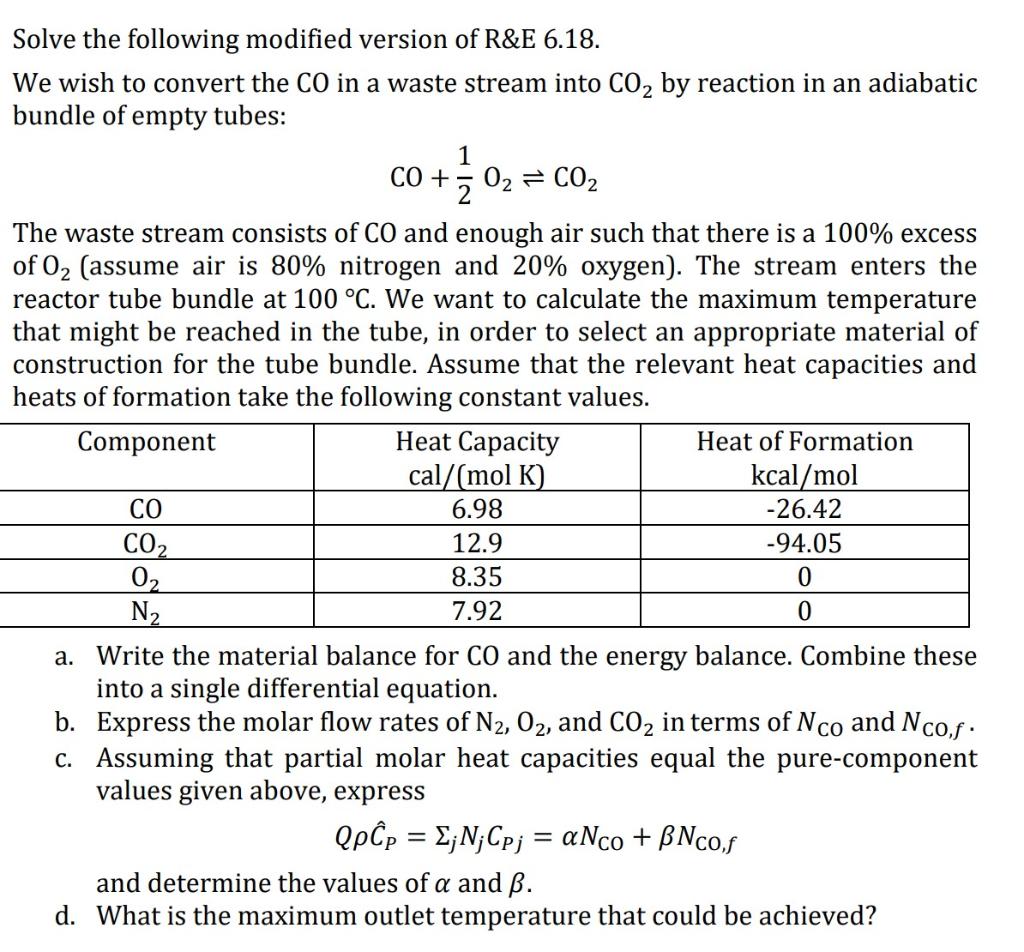
ANSWER ALL PARTS OF THE SINGLE QUESTION (A)-(D)
Solve the following modified version of R&E 6.18. We wish to convert the CO in a waste stream into CO2 by reaction in an adiabatic bundle of empty tubes: 1 CO + 2 02 02 = CO2 The waste stream consists of CO and enough air such that there is a 100% excess of O2 (assume air is 80% nitrogen and 20% oxygen). The stream enters the reactor tube bundle at 100 C. We want to calculate the maximum temperature that might be reached in the tube, in order to select an appropriate material of construction for the tube bundle. Assume that the relevant heat capacities and heats of formation take the following constant values. Component Heat Capacity Heat of Formation cal/(mol K) kcal/mol 6.98 -26.42 12.9 -94.05 02 8.35 0 N2 7.92 0 a. Write the material balance for CO and the energy balance. Combine these into a single differential equation. b. Express the molar flow rates of N2, O2, and CO2 in terms of Nco and Ncof. C. Assuming that partial molar heat capacities equal the pure-component values given above, express Qpp = ;N; Cpj = anco + BNCof and determine the values of a and . d. What is the maximum outlet temperature that could be achieved? CO2 =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


