Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer all please !! 15. For each of the following ions: a. Draw ALL reasonable resonance forms. Include all curved arrows for full credit. b.
answer all please !! 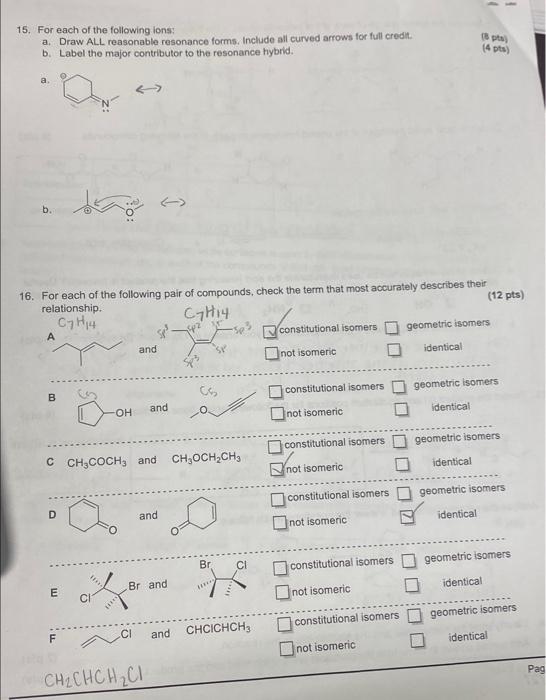
15. For each of the following ions: a. Draw ALL reasonable resonance forms. Include all curved arrows for full credit. b. Label the major contributor to the resonance hybrid. (8) pltw) (4 pts) a. b. 16. For each of the following pair of compounds, check the term that most accurately describes their relationship. and C7H+4 8 and constitutional isomers geometric isomers C CH3COCH3 and CH3OCH2CH3 constitutional isomers geometric isomers: D and constitutional isomers geometric isomers not isomeric identical E constitutional isomers geometric isomers not isomeric identical F and CHClCHCH3 constitutional isomers. geometric isomers CH2CHCHH2Cl 15. For each of the following ions: a. Draw ALL reasonable resonance forms. Include all curved arrows for full credit. b. Label the major contributor to the resonance hybrid. (8) pltw) (4 pts) a. b. 16. For each of the following pair of compounds, check the term that most accurately describes their relationship. and C7H+4 8 and constitutional isomers geometric isomers C CH3COCH3 and CH3OCH2CH3 constitutional isomers geometric isomers: D and constitutional isomers geometric isomers not isomeric identical E constitutional isomers geometric isomers not isomeric identical F and CHClCHCH3 constitutional isomers. geometric isomers CH2CHCHH2Cl 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


