Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ANSWER ALL QUESTIONS !! A 0.6 kg aluminum container that contains 0.350 L water is heated. Determine the total heat required in KJ for increasing

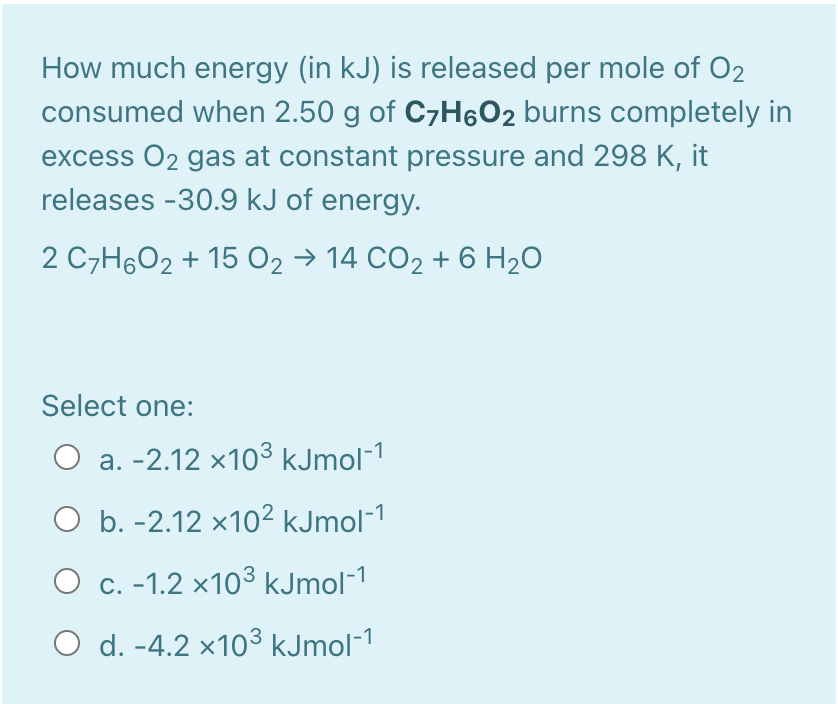
ANSWER ALL QUESTIONS !!
A 0.6 kg aluminum container that contains 0.350 L water is heated. Determine the total heat required in KJ for increasing the temperature of both container and water from 25C to 75C. The specific heat capacity of water is 4182 J/(kgC). The specific heat capacity of aluminum is 900 J/(kgC). Select one: O a. 100.255 O b. 272.01 O c. 800.21 O d. 25.631 How much energy (in kJ) is released per mole of O2 consumed when 2.50 g of CyH6O2 burns completely in excess O2 gas at constant pressure and 298 K, it releases -30.9 kJ of energy. 2 C7H6O2 + 15 O2 14 CO2 + 6 H2O Select one: O a. -2.12 x103 kJmol-1 O b. -2.12 x102 kJmol-1 O c. -1.2 x103 kJmol-1 O d. -4.2 x103 kJmol-1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


