Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ANSWER ALL QUESTIONS !! Choose the correct answer Select one: a. In a chemical reaction that absorbs energy, heat flows from surroundings to system whereas,

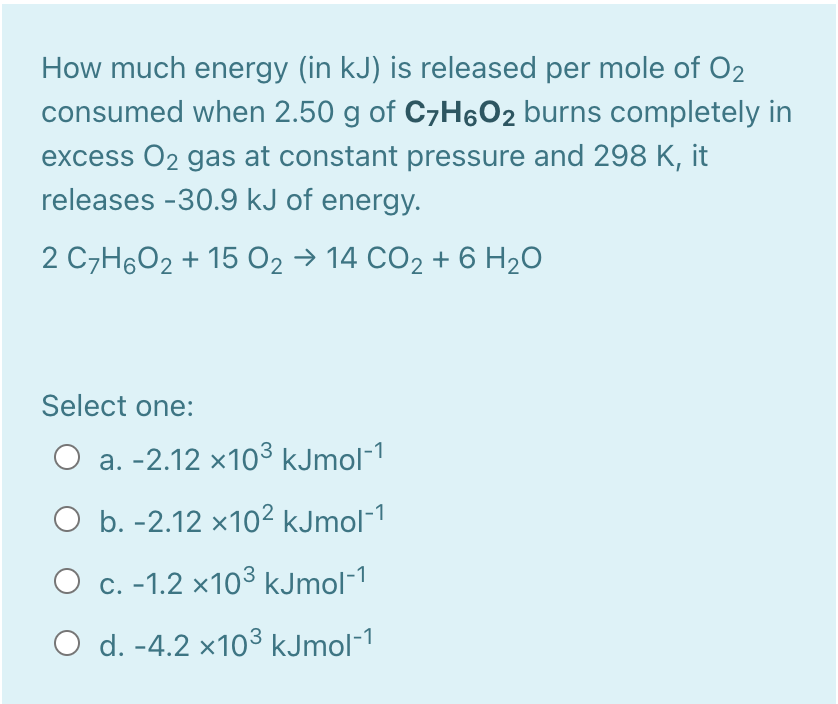
ANSWER ALL QUESTIONS !!
Choose the correct answer Select one: a. In a chemical reaction that absorbs energy, heat flows from surroundings to system whereas, heat flows from surroundings to system if chemical reaction releases energy O b. In a chemical reaction that absorbs energy, heat flows from system to surroundings whereas, heat flows from system to surroundings if chemical reaction releases energy c. In a chemical reaction that absorbs energy, heat flows from surroundings to system whereas, heat flows from system to surroundings if chemical reaction releases energy d. In a chemical reaction that absorbs energy, heat flows from system to surroundings whereas, heat flows from surroundings to system if chemical reaction releases energy O e. None of other answers is correct How much energy (in kJ) is released per mole of O2 consumed when 2.50 g of CyH6O2 burns completely in excess O2 gas at constant pressure and 298 K, it releases -30.9 kJ of energy. 2 C7H6O2 + 15 O2 14 CO2 + 6 H2O Select one: O a. -2.12 x103 kJmol-1 O b. -2.12 x102 kJmol-1 O c. -1.2 x103 kJmol-1 O d. -4.2 x103 kJmol-1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


