Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ANSWER ALL QUESTIONS !! Determine the amount of heat produced by adding 50.0 mL of 0.50 M HCl(aq) to 50.0 mL of 0.50 M NaOH(aq)

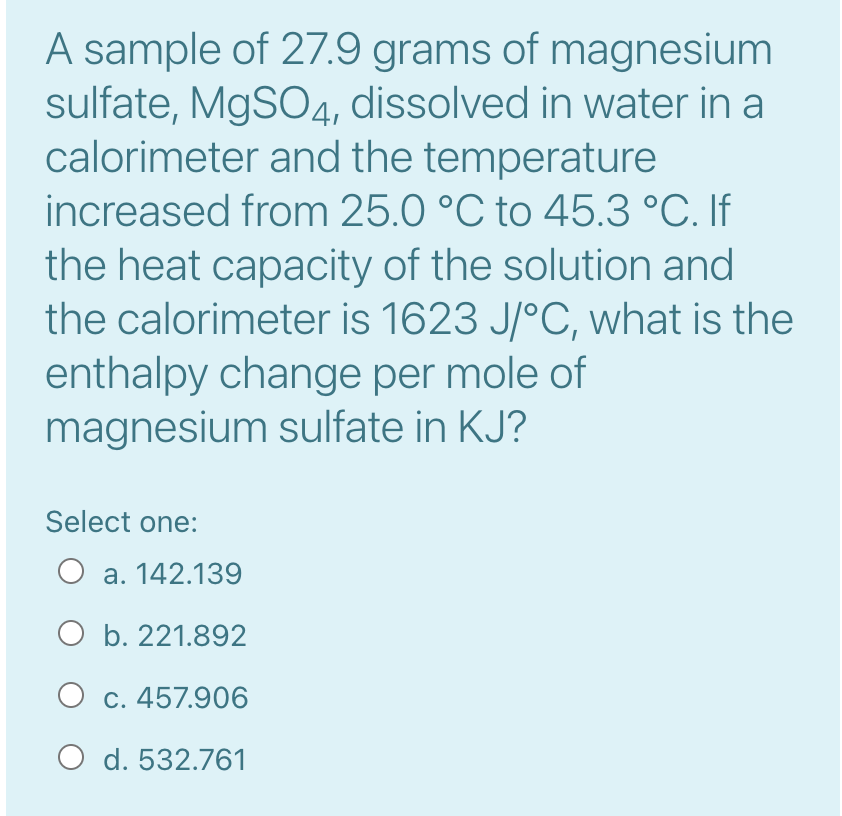
ANSWER ALL QUESTIONS !!
Determine the amount of heat produced by adding 50.0 mL of 0.50 M HCl(aq) to 50.0 mL of 0.50 M NaOH(aq) in a calorimeter, both at 25.0 C, when the temperature of the mixture reaches a maximum of 31.7 C. HCl(aq) + NaOH(aq) NaCl(aq) + H2 O(1) The specific heat capacity of water is 4.18 J /gC. Select one: O a. -6.9 KJ O b. 4.5 KJ O C. -2.8 Kg d. 11.3 KJ A sample of 27.9 grams of magnesium sulfate, MgSO4, dissolved in water in a calorimeter and the temperature increased from 25.0 C to 45.3 C. If the heat capacity of the solution and the calorimeter is 1623 J/C, what is the enthalpy change per mole of magnesium sulfate in KJ? Select one: a. 142.139 O b. 221.892 O c. 457.906 O d. 532.761Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


