Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer all The following elementary steps have been proposed for a reaction. H2O2+IH2O+IO(slow)H2O2+IOH2O+O2+I Does the overall reaction for this mechanism appear to be catalyzed? yes
answer all 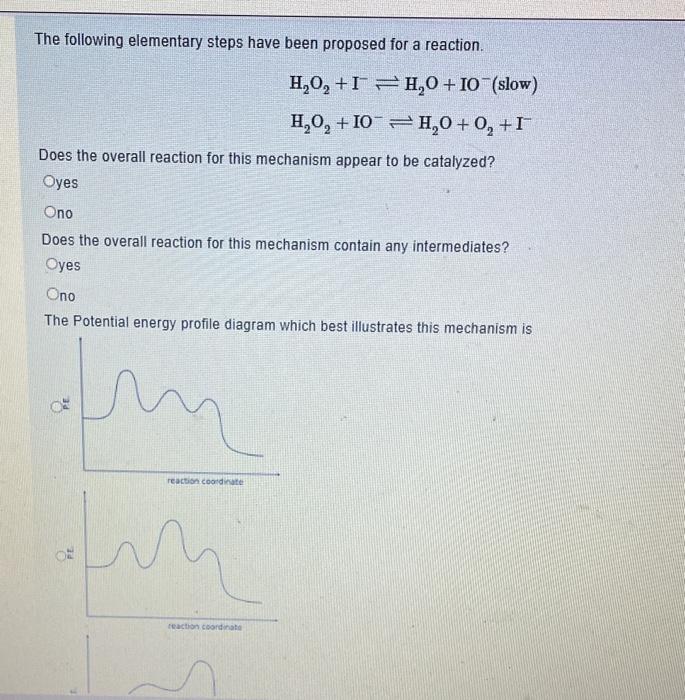
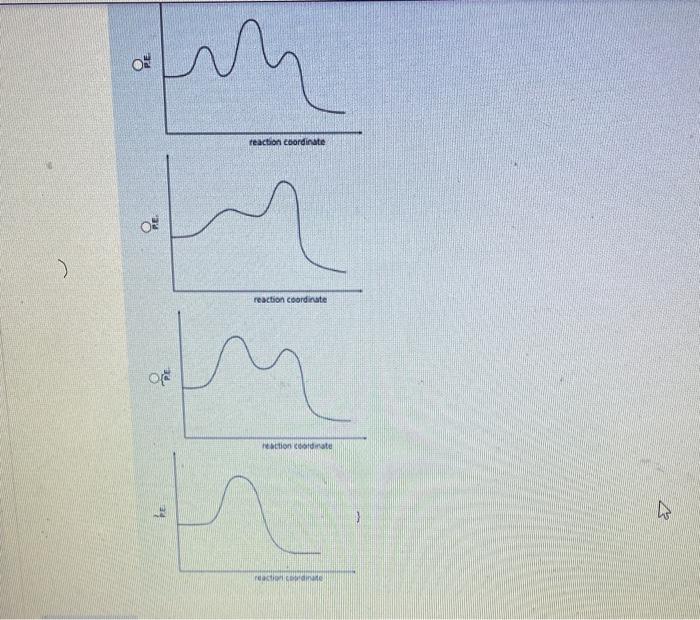
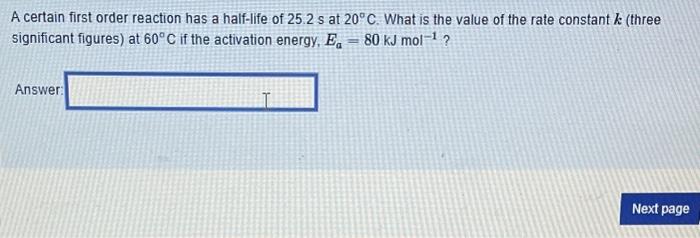
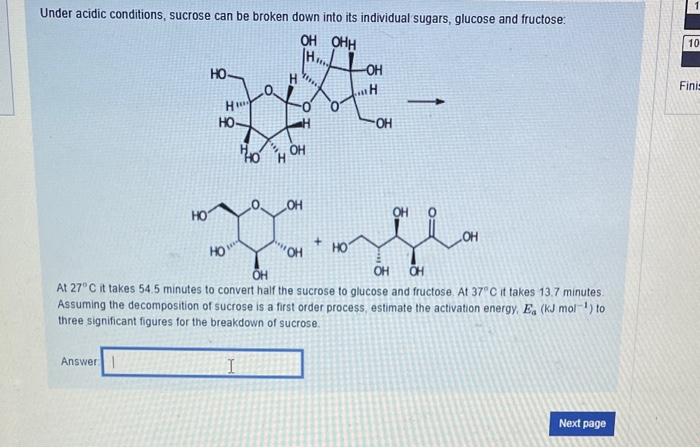
The following elementary steps have been proposed for a reaction. H2O2+IH2O+IO(slow)H2O2+IOH2O+O2+I Does the overall reaction for this mechanism appear to be catalyzed? yes no Does the overall reaction for this mechanism contain any intermediates? yes no The Potential energy profile diagram which best illustrates this mechanism is A certain first order reaction has a half-life of 25.2s at 20C. What is the value of the rate constant k (three significant figures) at 60C if the activation energy, Ea=80kJmol1 ? Answer: Under acidic conditions, sucrose can be broken down into its individual sugars, glucose and fructose: At 27C it takes 54.5 minutes to convert half the sucrose to glucose and fructose. At 37C it takes 13.7 minutes. Assuming the decomposition of sucrose is a first order process, estimate the activation energy, Ea(kJmol1) to three significant figures for the breakdown of sucrose 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


