Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer all the questions from 3 to 9 8:24 AM ...2.2KB/s l Vo LTE 86 CHEMISTRY assign... CHEMICAL THERMODYNAMICS OBJECTIVE CHEMISTRY IR Two mole of
Answer all the questions from 3 to 9
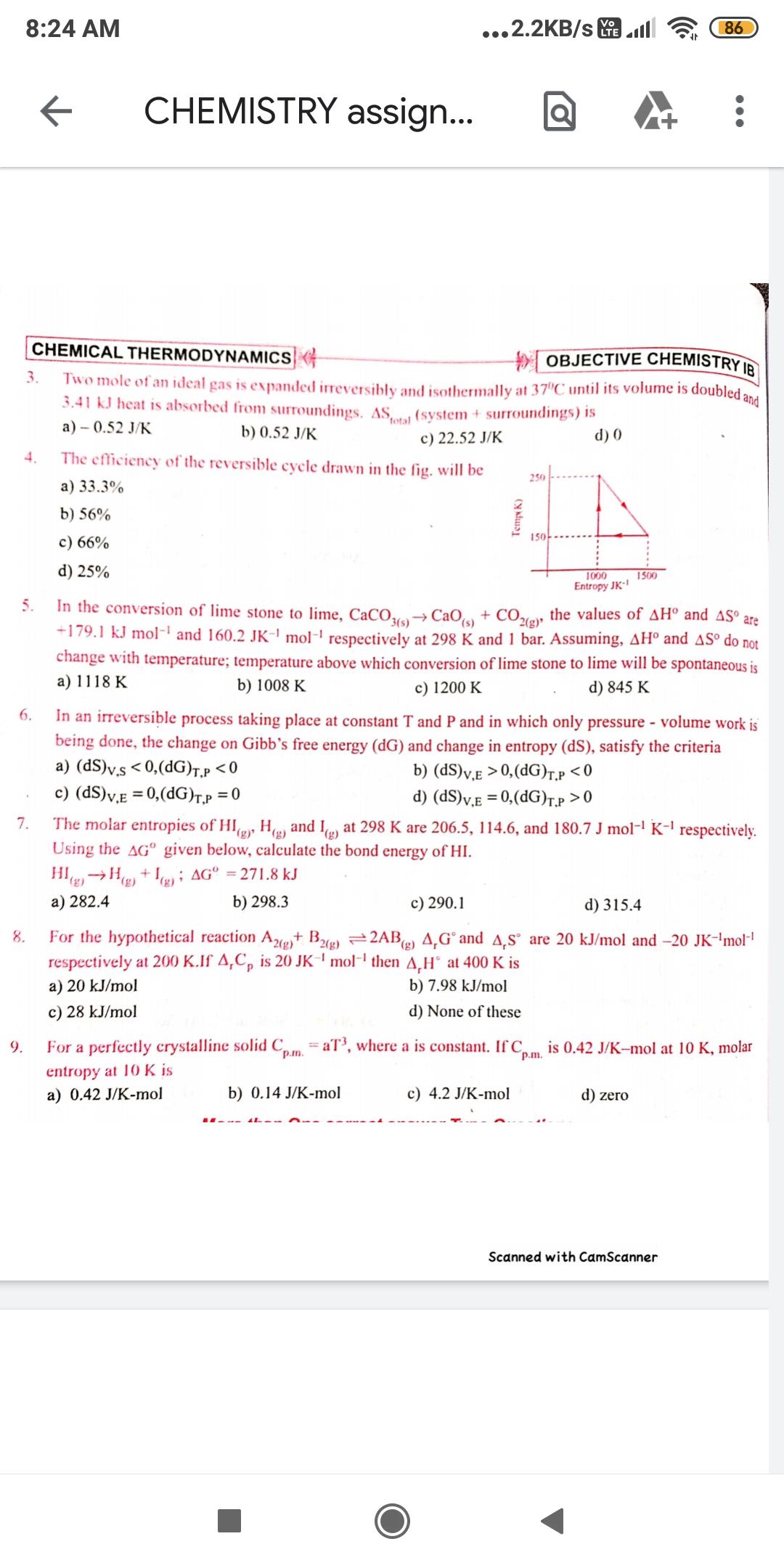
8:24 AM ...2.2KB/s l Vo LTE 86 CHEMISTRY assign... CHEMICAL THERMODYNAMICS OBJECTIVE CHEMISTRY IR Two mole of an ideal gas is expanded irreversibly and isothermally at 37C until its volume is doubled and 3.41 kJ heat is absorbed from surroundings. AS, (system + surroundings) is 3. total a) 0.52 J/K b) 0.52 J/K c) 22.52 J/K The efficiency of the reversible cycle drawn in the fig. will be d) 0 4. 250 a) 33.3% b) 56% 150 c) 66% d) 25% 1000 1500 Entropy JK In the conversion of lime stone to lime, CaCO19 CaOs) + CO2(g)" +179.1 kJ mol and 160.2 JK-' mol-' respectively at 298 K and 1 bar. Assuming, AH and AS do not change with temperature; temperature above which conversion of lime stone to lime will be spontaneous is a) 1118 K 5. the values of AH and AS are b) 1008 K c) 1200 K d) 845 K 6. In an irreversible process taking place at constant T and P and in which only pressure volume work is being done, the change on Gibb's free energy (dG) and change in entropy (dS), satisfy the criteria a) (dS)v.s < 0,(dG)T.P 0,(dG)T.p 0 The molar entropies of HI, He) and I at 298 K are 206.5, 114.6, and 180.7 J mol- K-l respectively. Using the AG given below, calculate the bond energy of HI. 7. (g) HIgH, + Ig); AG = 271.8 kJ (3), a) 282.4 b) 298.3 c) 290.1 d) 315.4 For the hypothetical reaction A2+ B2) =2AB) 4,G and A,S are 20 kJ/mol and -20 JK-'mol respectively at 200 K.If A,C, is 20 JK' mol- then A,H at 400 K is 8. a) 20 kJ/mol b) 7.98 kJ/mol c) 28 kJ/mol d) None of these For a perfectly crystalline solid C aT, where a is constant. If C. p.m. is 0.42 J/K-mol at 10 K, molar 9. p.m. entropy at 10 K is a) 0.42 J/K-mol b) 0.14 J/K-mol c) 4.2 J/K-mol d) zero Scanned with CamScanner Temp K)
Step by Step Solution
★★★★★
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


