Question
Given the data in the table below, AHrxn for the reaction 2Ag25 (s) + 02 (8)+ 2Ag20 (6) + 25 (s) is kJ. Substance
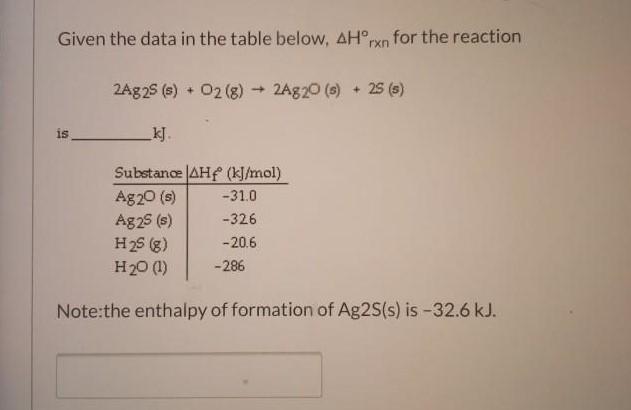
Given the data in the table below, AHrxn for the reaction 2Ag25 (s) + 02 (8)+ 2Ag20 (6) + 25 (s) is kJ. Substance AHf (kJ/mol) Ag20 (s) Ag25 (s) H25 (g) H20 (1) -31.0 -326 -20.6 -286 Note:the enthalpy of formation of Ag2S(s) is-32.6 kJ.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Statistics Exploring The World Through Data
Authors: Robert Gould, Colleen Ryan
2nd Edition
9780321978509, 321978277, 321978501, 978-0321978271
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



