Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer and show all work please 1. Which of the following elements exist as diatomic molecules? a. chlorine b. potassium c. silver d. oxygen 2.
answer and show all work please 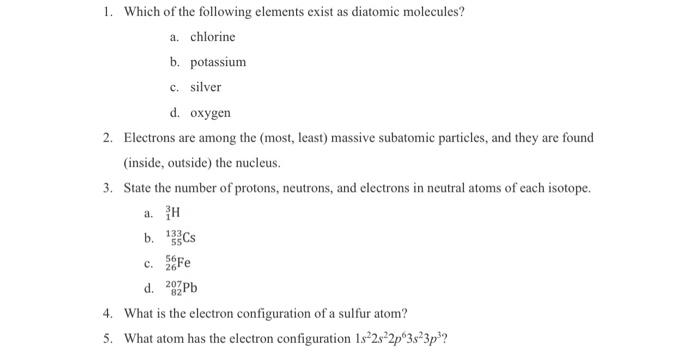
1. Which of the following elements exist as diatomic molecules? a. chlorine b. potassium c. silver d. oxygen 2. Electrons are among the (most, least) massive subatomic particles, and they are found (inside, outside) the nucleus. 3. State the number of protons, neutrons, and electrons in neutral atoms of each isotope. a. 13H b. 55133Cs c. 2656Fe d. 82207Pb 4. What is the electron configuration of a sulfur atom? 5. What atom has the electron configuration 1s22s22p63s23p3 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


