Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer both fast pls Referencer Use the References to access important values if needed for this question. pl pls M ipt Tpl pl 2 The
answer both fast pls 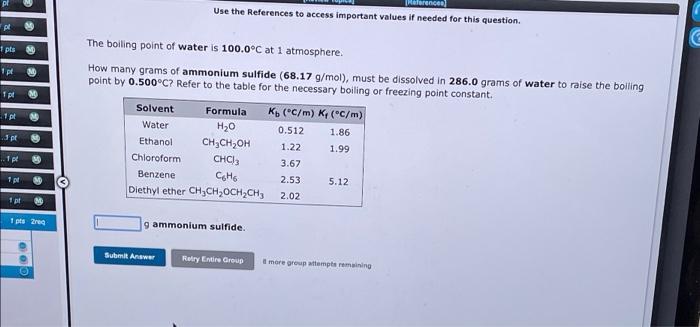
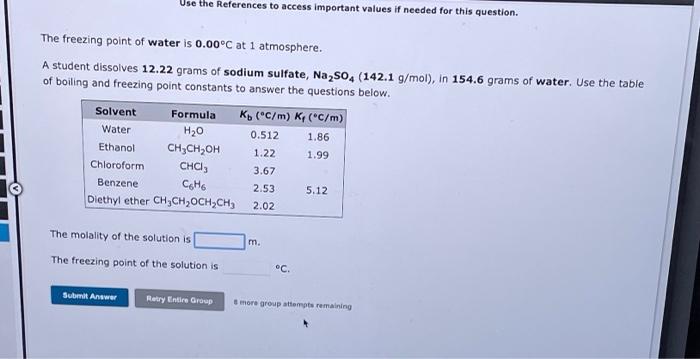
Referencer Use the References to access important values if needed for this question. pl pls M ipt Tpl pl 2 The boiling point of water is 100.0C at 1 atmosphere. How many grams of ammonium sulfide (68.17 g/mol), must be dissolved in 286.0 grams of water to raise the boiling point by 0.500C? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula Ko (C/m) K(C/m) Water H20 0.512 1.86 Ethanol ,, 1.22 1.99 Chloroform CHC) 3.67 Benzene Coho 2.53 5.12 Diethyl ether CH CH, OCH2CH3 2.02 3 pt 1 p M 1 pt M 1 pts reg gammonium sulfide. Submit Answer Relry Entire Group more group attempts remaining Use the References to access important values if needed for this question. The freezing point of water is 0.00C at 1 atmosphere. A student dissolves 12.22 grams of sodium sulfate, Na2SO4 (142.1 g/mol), in 154.6 grams of water. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula K(C/m) K (C/m) Water H20 0.512 1.86 Ethanol CH3CH2OH 1.22 Chloroform CHCI, 3.67 Benzene Coto 2.53 5.12 Diethyl ether CH CH,OCH2CH) 1.99 2.02 The molality of the solution is The freezing point of the solution is C. Submit Answer Retry Entire Group more group attempts remaining 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


