Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer both please need help For the following reaction what is the correct expression for K? N2 (g) + 3Br2(g) = 2NBrz(s) a. Kc =
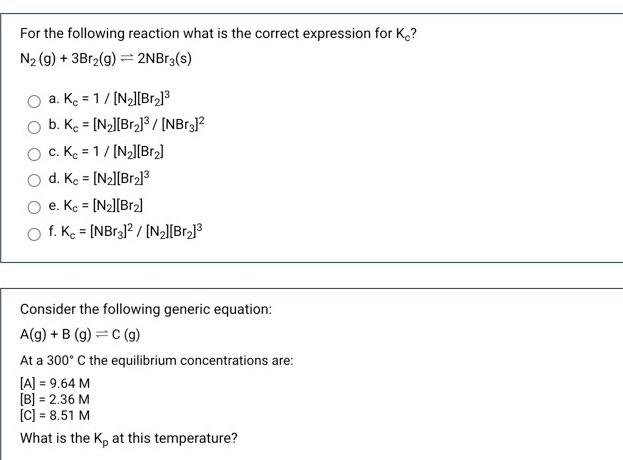
answer both please need help
For the following reaction what is the correct expression for K? N2 (g) + 3Br2(g) = 2NBrz(s) a. Kc = 1/(N_][Br] b. Kc = [N_][Br]3/[NBr312 c. Kc = 1/[N][Br] d. Kc = [N2][Br.] O e. Kc = [N][Br] f. Kc = [NBr312 / [NZ][Br.] Consider the following generic equation: A(g) + B (9)=C(9) At a 300C the equilibrium concentrations are: [A] = 9.64 M [B] = 2.36 M [C] = 8.51 M What is the ke at this temperature Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


