Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer DCu = 2.3*10^-9 DZn = 5.2*10^-9 5.4 A block of copper was joined to a block of -brass ( 30wt%Zn,70wt%Cu ) with inert markers
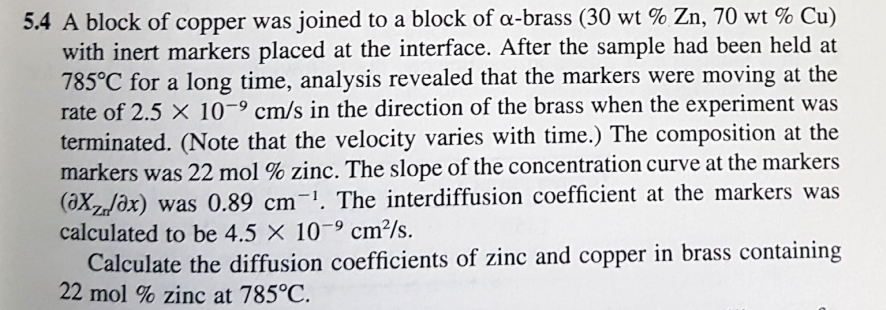
Answer DCu = 2.3*10^-9 DZn = 5.2*10^-9
5.4 A block of copper was joined to a block of -brass ( 30wt%Zn,70wt%Cu ) with inert markers placed at the interface. After the sample had been held at 785C for a long time, analysis revealed that the markers were moving at the rate of 2.5109cm/s in the direction of the brass when the experiment was terminated. (Note that the velocity varies with time.) The composition at the markers was 22mol% zinc. The slope of the concentration curve at the markers (Xz/x) was 0.89cm1. The interdiffusion coefficient at the markers was calculated to be 4.5109cm2/s. Calculate the diffusion coefficients of zinc and copper in brass containing 22mol% zinc at 785CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


