Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer directly. No solutions needed 1.) 2.) 3.) A compound contains 0.621gC,0.104gH,0.362gN, and 0.413g of 0 . In a separate experiment, its molar mass is
Answer directly. No solutions needed
1.)
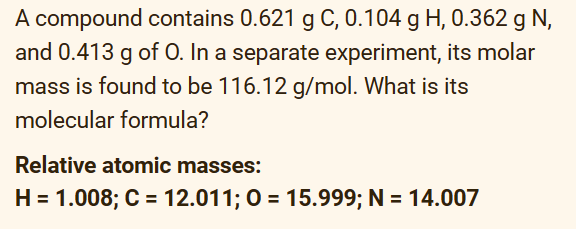
2.)

3.)
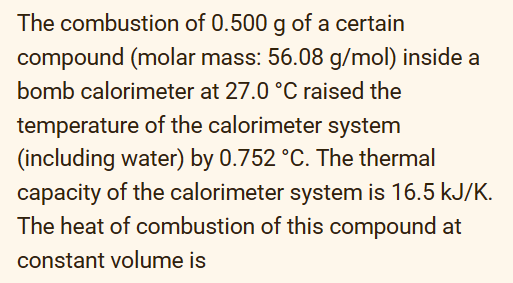
A compound contains 0.621gC,0.104gH,0.362gN, and 0.413g of 0 . In a separate experiment, its molar mass is found to be 116.12g/mol. What is its molecular formula? Relative atomic masses: H=1.008;C=12.011;O=15.999;N=14.007 In the reaction of metal M below 4M(s)+5O2(g)2M2O5(s)H=2256kJ what is the standard enthalpy change of formation of M2O5 ? Select one: a. 4512kJ/mol b. 1128kJ/mol c. 2256kJ/mol d. 564.0kJ/mol The combustion of 0.500g of a certain compound (molar mass: 56.08g/mol ) inside a bomb calorimeter at 27.0C raised the temperature of the calorimeter system (including water) by 0.752C. The thermal capacity of the calorimeter system is 16.5kJ/K. The heat of combustion of this compound at constant volume is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


