Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer each and every question in one hour please and make sure all the solution is correct. Post-Lab Questions: 1. Does your experimental density of
answer each and every question in one hour please and make sure all the solution is correct. 
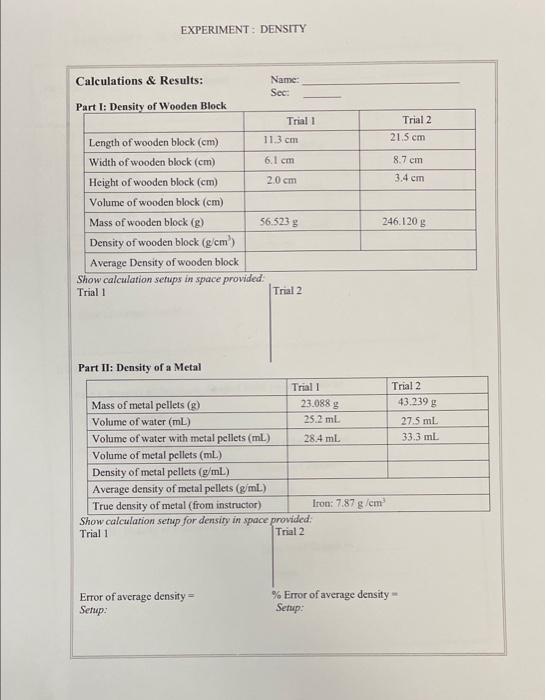
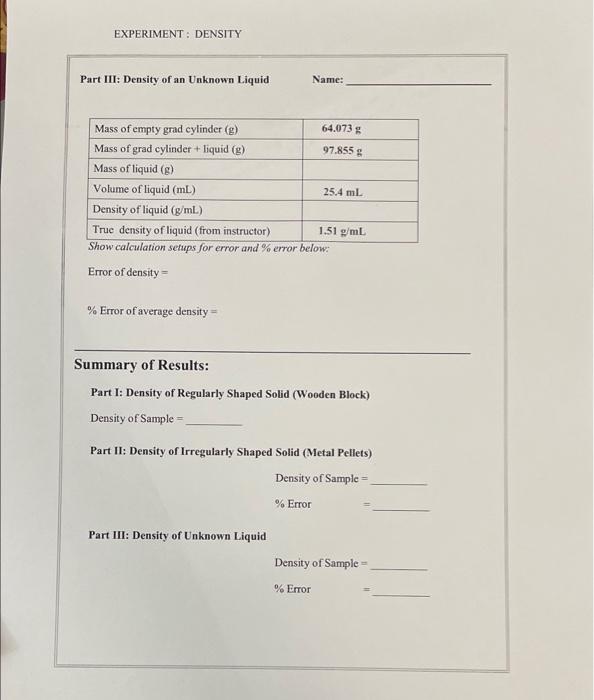
Post-Lab Questions: 1. Does your experimental density of the metal sample agree with the true value? If no, give reasons for the discrepancy. Do not include operator errors. 2. Does your experimental density of the unknown liquid agree with the true value? If no, give reasons for the discrepancy. Do not include operator errors. 3. What is the density in g/mL of a ball that has a mass of 22.1 ounces and a diameter of 2.04 inches? Will it float if placed in a bucket of water? The answer is "no." Provide the calculations to prove this and explain in full sentences. 4. If there were air bubbles trapped under the metal pellets in Part II when you recorded its volume, would that give you an experimental density that is too high or too low? Explain. Would this be considered instrumental, method or operator error? Explain. 5. If you were to mix your unknown liquid in Part III with water, would you expect it to float or sink? Explain. EXPERIMENT: DENSITY Trial 2 21.5 cm 8.7 cm 3.4 cm Calculations & Results: Name: Sec: Part I: Density of Wooden Block Trial 1 11.3 cm Length of wooden block (cm) Width of wooden block (cm) 6.1 cm Height of wooden block (cm) 2.0 cm Volume of wooden block (cm) Mass of wooden block (2) 56,523 g Density of wooden block (g/cm) Average Density of wooden block Show calculation setups in space provided Trial 1 Trial 2 246.120 g Trial 2 43.239 g 27.5 mL 33.3 mL Part II: Density of a Metal Trial 1 Mass of metal pellets (8) 23.088 g Volume of water (mL) 25.2 ml Volume of water with metal pellets (ml.) 28.4 ml Volume of metal pellets (mL) Density of metal pellets (g/mL) Average density of metal pellets (g/mL) True density of metal (from instructor) Iron: 7.87 g/cm Show calculation setup for density in space provided: Trial 1 Trial 2 Error of average density Setup % Error of average density Setup: EXPERIMENT: DENSITY Part III: Density of an Unknown Liquid Name: Mass of empty grad cylinder (p) 64.073 Mass of grad cylinder + liquid (g) 97.855 g Mass of liquid (g) Volume of liquid (mL) 25.4 mL Density of liquid (g/ml.) True density of liquid (from inst Actor) 1.51 g/mL. Show calculation setups for error and % error below: Error of density = % Error of average density = Summary of Results: Part 1: Density of Regularly Shaped Solid (Wooden Block) Density of Sample Part II: Density of Irregularly Shaped Solid (Metal Pellets) Density of Sample % Error Part III: Density of Unknown Liquid Density of Sample - % Error 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


