Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer each of the following questions 8. For each of the following pairs of electrons, indicate whether or not they could both be in 8.
Answer each of the following questions 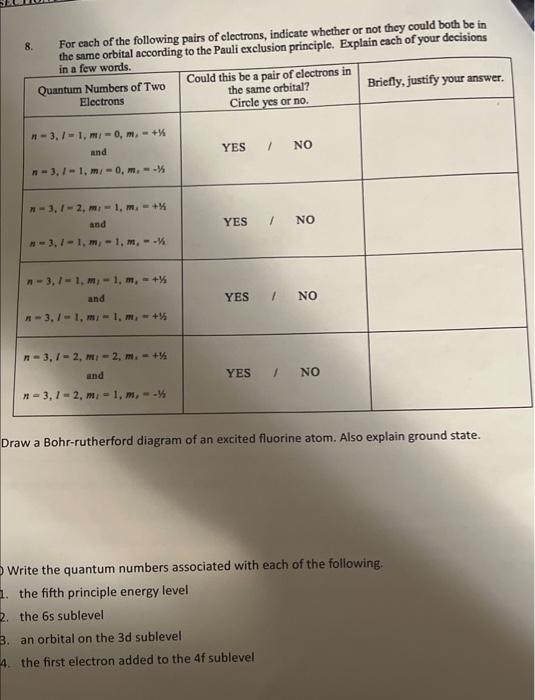
8. For each of the following pairs of electrons, indicate whether or not they could both be in 8. For cach of the following pairs of clectrons, indicate whether or not they could both be in Draw a Bohr-rutherford diagram of an excited fluorine atom. Also explain ground state. Write the quantum numbers associated with each of the following. 1. the fifth principle energy level 2. the 6 s sublevel 3. an orbital on the 3d sublevel 4. the first electron added to the 4 f sublevel 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


