Question
5.35 gms of NH4Clis added to 1 lit of 0.4 M NH4OHsolution. Find the [OH Jof the resulting solution assuming no change in volume
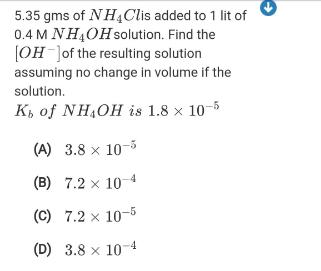
5.35 gms of NH4Clis added to 1 lit of 0.4 M NH4OHsolution. Find the [OH Jof the resulting solution assuming no change in volume if the solution. K, of NH,OH is 1.8 x 10-5 (A) 3.8 x 10-5 (B) 7.2 x 10-4 (C) 7.2 x 10-5 (D) 3.8 x 104
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial and Managerial Accounting the basis for business decisions
Authors: Jan Williams, Susan Haka, Mark Bettner, Joseph Carcello
17th edition
007802577X, 978-0078025778
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



