Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer oral courses 27845 Jodie Question 2 Given the following information: 3/2 A2+ B(g) + C(g) AC(g) + A2B(g) K, 2A(g) +B(g) AB(g) K2 2A(g)
answer 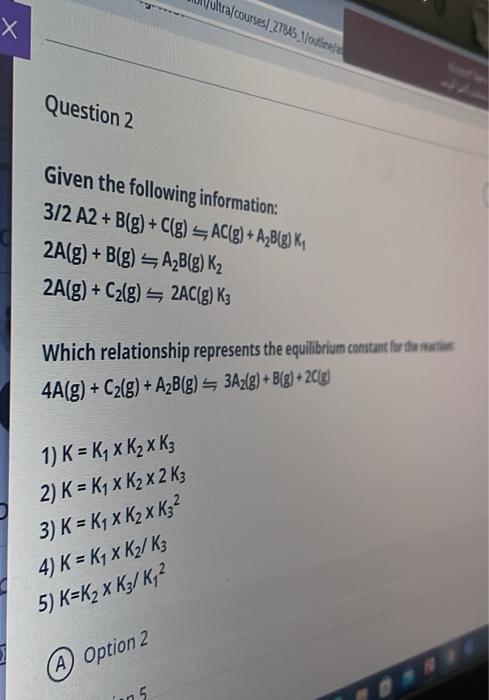
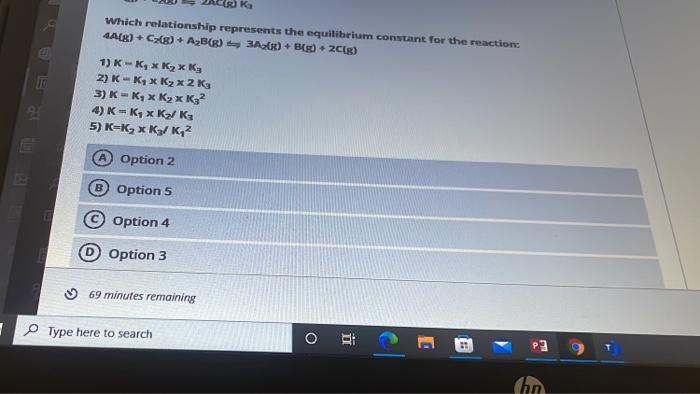
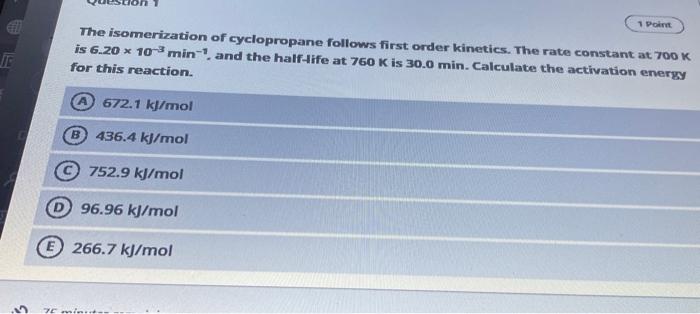
oral courses 27845 Jodie Question 2 Given the following information: 3/2 A2+ B(g) + C(g) AC(g) + A2B(g) K, 2A(g) +B(g) AB(g) K2 2A(g) + C2(g) 2AC(g) K3 Which relationship represents the equilibrium constant for the 4A(g) + C2(g) + A2B(g) 342(g) + B[g) + 2C(g) 1) K = Ky x K x K3 2) K = K4 x K x 2 K3 3) K = K4 x K2 K32 4) K = K4 x K2 K3 5) K=K2 x K3/K, A Option 2 25 s Which relationship represents the equilibrium constant for the reaction 4A(8) + C(8) + AB6) 3A) + B + 2C[g) 1) -, K 2) K-K, x K x 2 K3 3) K - K x K x K? 4) K = Ky x K / ks 5) K=K3 x K/K,? Option 2 B Option 5 Option 4 0 Option 3 69 minutes remaining Type here to search O et Chn 1 Point The isomerization of cyclopropane follows first order kinetics. The rate constant at 700 K is 6.20 * 10 min-and the half-life at 760 K is 30.0 min. Calculate the activation energy for this reaction. A 672.1 kJ/mol B 436.4 kj/mol 752.9 kJ/mol 96.96 kJ/mol 266.7 kJ/mol 5 20 min 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


