Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer please!!! Question 4 (1 point) How many grams of antimony can be produced from 41.3 g of antimony(Ill) oxide, Sb406, according to the equation
answer please!!! 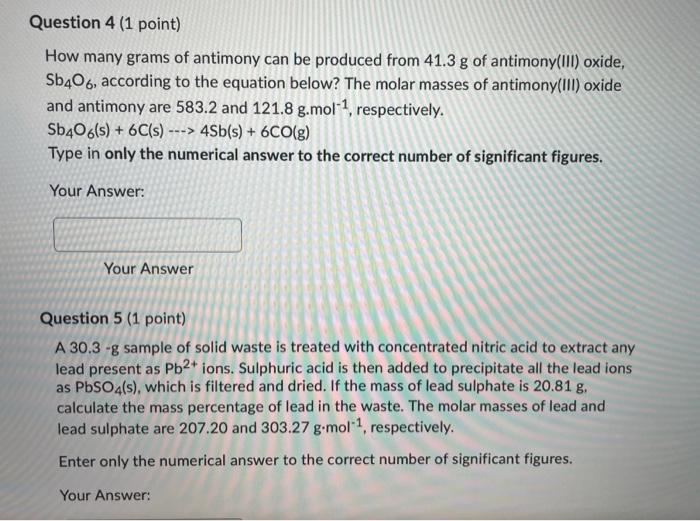
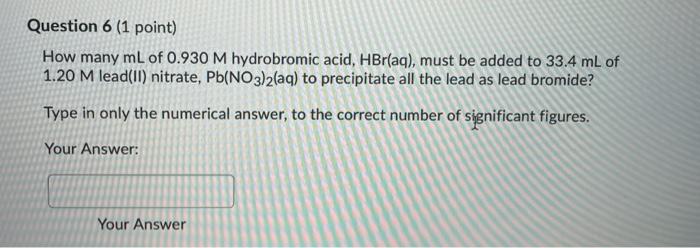
Question 4 (1 point) How many grams of antimony can be produced from 41.3 g of antimony(Ill) oxide, Sb406, according to the equation below? The molar masses of antimony(III) oxide and antimony are 583.2 and 121.8 g.mol-1, respectively. Sb406(s) + 6C(s) ---> 4Sb(s) + 6CO(g) Type in only the numerical answer to the correct number of significant figures. Your Answer: Your Answer Question 5 (1 point) A 30.3 -g sample of solid waste is treated with concentrated nitric acid to extract any lead present as Pb2+ ions. Sulphuric acid is then added to precipitate all the lead ions as PbSO4(s), which is filtered and dried. If the mass of lead sulphate is 20.81 g. calculate the mass percentage of lead in the waste. The molar masses of lead and lead sulphate are 207.20 and 303.27 g.mol-1, respectively. Enter only the numerical answer to the correct number of significant figures. Your Answer: Question 6 (1 point) How many mL of 0.930 M hydrobromic acid, HBr(aq), must be added to 33.4 ml of 1.20 M lead(II) nitrate, Pb(NO3)2(aq) to precipitate all the lead as lead bromide? Type in only the numerical answer to the correct number of significant figures. Your Answer: Your 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


